Chapter 18 OXYGEN
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
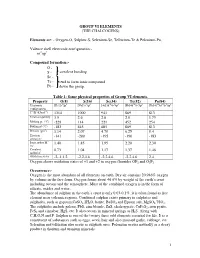
Group Vi Elements (The Chalcogens)
GROUP VI ELEMENTS (THE CHALCOGENS) Elements are: - Oxygen-O, Sulphur-S, Selenium-Se, Tellurium-Te & Polonium-Po. Valence shell electronic configuration:- ns2np4 Compound formation:- O - S - covalent bonding Se - Te - tend to form ionic compound Po - down the group. Table 1: Some physical properties of Group VI elements. Property O(8) S(16) Se(34) Te(52) Po(84) Electronic [He]2s22p4 [Ne]3s23p4 [Ar]3d104s24p4 [Kr]4d105s25p4 [Xe]4f145d106s26p4 configuration 1st IE (kJmol-1) 1314 1000 941 869 813 Electronegativity 3.5 2.6 2.6 2.0 1.75 Melting pt. (oC) -229 114 221 452 254 Boiling pt (oC) -183 445 685 869 813 Density (gm-3) 1.14 2.07 4.79 6.25 9.4 Electron -141 -200 -195 -190 -183 affinity,E- Ionic radius M2- 1.40 1.85 1.95 2.20 2.30 /Ao Covalent 0.73 1.04 1.17 1.37 1.46 radius/Ao Oxidation states -2,-1,1,2 -2,2,4,6 -2,2,4,6 -2,2,4,6 2,4 Oxygen shows oxidation states of +1 and +2 in oxygen fluorides OF2 and O2F2 Occurrence:- Oxygen is the most abundant of all elements on earth. Dry air contains 20.946% oxygen by volume in the free form. Oxygen forms about 46.6% by weight of the earth’s crust including oceans and the atmosphere. Most of the combined oxygen is in the form of silicate, oxides and water. The abundance of sulphur in the earth’s crust is only 0.03-0.1%. it is often found as free element near volcanic regions. -

Effect of Nitrogen/Oxygen Substances on the Pyrolysis of Alkane-Rich Gases to Acetylene by Thermal Plasma
energies Article Effect of Nitrogen/Oxygen Substances on the Pyrolysis of Alkane-Rich Gases to Acetylene by Thermal Plasma Wei Huang, Junkui Jin, Guangdong Wen, Qiwei Yang * ID , Baogen Su * and Qilong Ren Key Laboratory of Biomass Chemical Engineering, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, China; [email protected] (W.H.); [email protected] (J.J.); [email protected] (G.W.); [email protected] (Q.R.) * Correspondence: [email protected] (Q.Y.); [email protected] (B.S.); Tel.: +86-571-87951125 (Q.Y.) Received: 23 December 2017; Accepted: 29 January 2018; Published: 2 February 2018 Abstract: It is important to convert alkane-rich gases, such as coke oven gas, to value-added chemicals rather than direct emission or combustion. Abundant nitrogen/oxygen substances are present in the actual alkane-rich gases. However, the research about how they influence the conversion in the pyrolysis process is missing. In this work, a systematic investigation on the effect of various nitrogen/oxygen-containing substances, including N2, CO, and CO2,on the pyrolysis of CH4 to C2H2 was performed by a self-made 50 kW rotating arc thermal plasma reactor, and the pyrolysis of a simulated coke oven gas as a model of alkane-rich mixing gas was conducted as well. It was found that the presence of N2 and CO2 was not conducive to the main reaction of alkane pyrolysis for C2H2, while CO, as a stable equilibrium product, had little effect on the cracking reaction. Consequently, it is suggested that a pretreatment process of removing N2 and CO2 should be present before pyrolysis. -

Chm 303 Course Guide
COURSE GUIDE CHM 303 INORGANIC CHEMISTRY III Course Team Professor Phd Aliyu (Course Writer) - Department of Chemistry University of Abuja Prof. Sulaiman o. Idris (Course Reviewer) - Department of Chemistry Ahmadu Bello University Zaria Dr Emeka C. Ogoko - (Head of Department) - NOUN NATIONAL OPEN UNIVERSITY OF NIGERIA CHM 303 COURSE GUIDE © 2021 by NOUN Press National Open University of Nigeria Headquarters University Village Plot 91, Cadastral Zone NnamdiAzikiwe Expressway Jabi, Abuja Lagos Office 14/16 Ahmadu Bello Way Victoria Island, Lagos e-mail: [email protected] URL: www.nou.edu.ng All rights reserved. No part of this book may be reproduced, in any form or by any means, without permission in writing from the publisher. Printed 2021 ISBN: 978-978-058-092-6 ii CHM 303 COURSE GUIDE CONTENTS PAGE Introduction………………………………………………. iv What You Will Learn in This Course…………………….. iv Course Aim………………………………………………… iv Course Objectives…………………………………………. v Working through this Course……………………………… v The Course Materials……………………………………… v Study Sessions……………………………………………… vi Presentation Schedule……………………………………… vii Assessment………………………………………………… vii Tutor Marked Assignments ………………………………… vii Final Examination and Grading……………………………. vii Course Marking Scheme…………………………………… viii Facilitators/Tutors and Tutorials…………………………… viii iii CHM 303 COURSE GUIDE INTRODUCTION Inorganic Chemistry III course (CHM 303) is one of the core courses for the Bachelor of Science degree programme in Chemistry. It is a three- credit unit course at 300 level of the National Open University of Nigeria, designed for students with a fair background knowledge in inorganic Chemistry II course. This course gives an over view of the physical and chemical properties of the elements of the periodic table in addition to the extraction and purification of metals. -

Group 18 - the Elements
Group 18 - The Elements ! All found in minute quantities in air. • Argon is most abundant (and cheapest), comprising 0.934% of air by volume. ! Although rare on earth, helium is the second most abundant element in the universe (H, 76%; He, 23%), being a major component of stars. ! All radon isotopes are short-lived and are continuously being produced by natural decay processes. 222 • Longest lived isotope is Rn (á, t½ = 3.825 days). ! Condensed phases are held together by van der Waals forces, which increase smoothly down the group. Element b.p. (K) I (kJ/mol) He 4.18 2372 Ne 27.13 2080 Ar 87.29 1520 Kr 120.26 1351 Xe 166.06 1169 Rn 208.16 1037 Helium ! Helium is found in certain natural gas deposits (e.g., in Kansas), where it probably forms as a result of radioactive decay in the rocks. ! Because of its low boiling point, it is used widely in cryogenic applications. • Also used in airships (e.g., Goodyear blimp) and as a balloon filler. • Used as a fill gas for deep diving, because it is less soluble in blood than nitrogen, thus avoiding the "bends." ! The 4He isotope has the lowest know boiling point, 4.12 K, at which point it is called He-I. • On cooling to 2.178 K a phase transition to He-II occurs. • He-II has an expanded volume, almost no viscosity, and is superconducting. • He-II can readily flow uphill to equalize volumes in adjacent vessels. • It cannot be stored in glass Dewars, because it leaks through glass into the evacuated space, posing an explosion risk. -

United States Patent Office
Patented Feb. 21, 1928, 1,660,220 UNITED STATES PATENT OFFICE. ANTHONY-G. DE GOLYER, OF BROOKYN, NEW YORK. CoPPER BEFINING. NoDrawing. Application filed April 16, 1927. serial No. 184,443. My present invention relates to a new and copper, cement copper, scrap copper, etc. It improved process for the refining of copper, is also adapted for use in both the produc-65 and relates particularly to methods for the tion of cast shapes to be used in the produc production of copper which is entirely free tion of wire, tubes, sheets and other wrought from dissolved and occluded gas and from articles, and for the production of finished oxygen. or semi-finished castings in sand or other In response to industrial and technical de molds, PE, castings having high 60 mands, many attempts have been made to electrical conductivity, high tensile strength produce oxygen free copper by direct refin and excellent machining qualities. O ing methods and otherwise. While it has Before describing my invention I will been possible heretofore to produce oxygen give, for the purpose of comparison, an out free copper by means of previously proposed line of the pyrometallurgical method which 05 methods, copper so produced did not have has heretofore been generally used for the the necessary additional qualities of high refining of copper, and the aii Opera 5 metallic copper content, high electrical con tions required for the production of deoxi ductivity, and freedom from dissolved or dized copper. occluded gas which, latter, resulted in gas or Electrolytic copper in the form of cath-0 “blow holes in the solid copper. -

Elemental Fluorine Product Information (Pdf)
Elemental Fluorine Contents 1 Introduction ............................................................................................................... 4 2.1 Technical Application of Fluorine ............................................................................. 5 2.2 Electronic Application of Fluorine ........................................................................... 7 2.3 Fluorine On-Site Plant ............................................................................................ 8 3 Specifications ............................................................................................................ 9 4 Safety ...................................................................................................................... 10 4.1 Maintenance of the F2 system .............................................................................. 12 4.2 First Aid ................................................................................................................ 13 5.1 Chemical Properties ............................................................................................. 14 5.2 Physical Data ....................................................................................................... 15 6 Toxicity .................................................................................................................... 18 7 Shipping and Transport ........................................................................................... 20 8 Environment ........................................................................................................... -

Basic Keyword List
NOTICE TO AUTHORS 2008 Basic Keyword list A Antibodies Bismuth Chalcogens Ab initio calculations Antifungal agents Block copolymers Chaperone proteins Absorption Antigens Bond energy Charge carrier injection Acidity Antimony Bond theory Charge transfer Actinides Antisense agents Boranes Chelates Acylation Antitumor agents Borates Chemical vapor deposition Addition to alkenes Antiviral agents Boron Chemical vapor transport Addition to carbonyl com- Aqueous-phase catalysis Bridging ligands Chemisorption pounds Arene ligands Bromine Chemoenzymatic synthesis Adsorption Arenes Brønsted acids Chemoselectivity Aerobic oxidation Argon Chiral auxiliaries Aggregation Aromatic substitution C Chiral pool Agostic interactions Aromaticity C-C activation Chiral resolution Alanes Arsenic C-C bond formation Chirality Alcohols Arylation C-C coupling Chlorine Aldehydes Aryl halides C-Cl bond activation Chromates Aldol reaction Arynes C-Glycosides Chromium Alkali metals As ligands C-H activation Chromophores Alkaline earth metals Asymmetric amplification C1 building blocks Circular dichroism Alkaloids Asymmetric catalysis Cadmium Clathrates Alkanes Asymmetric synthesis Cage compounds Clays Alkene ligands Atmospheric chemistry Calcium Cleavage reactions Alkenes Atom economy Calixarenes Cluster compounds Alkylation Atropisomerism Calorimetry Cobalamines Alkyne ligands Aurophilicity Carbanions Cobalt Alkynes Autocatalysis Carbene homologues Cofactors Alkynylation Automerization Carbene ligands Colloids Allenes Autoxidation Carbenes Combinatorial chemistry Allosterism -

2018 Annual Survey of Biological and Chemical Agents Regulated by Homeland Security (And Carcinogens Regulated by OSHA)
Name: Dept: Date: 2018 Annual Survey of Biological and Chemical Agents regulated by Homeland Security (and carcinogens regulated by OSHA) Due (date) All labs that do not have a current chemical inventory in Chematix MUST complete this survey. The University is required to make an annual report of all chemicals on the Chemical Facility Anti-Terrorism Standards (CFATS) lists. Additional information regarding the regulations is available on the EH&S website at http://www.safety.rochester.edu/restricted/occsafe/chemicalagent.html and https://www.selectagents.gov. 1. Please review the lists on the following pages and indicate if any are possessed by your lab. The CAS# has been added to the list for ease of searching databases. The CAS# is a Chemical Abstract Service numbering system which assigns a unique number to every chemical substance based on structure; this helps avoid confusion by use of synonyms or different naming conventions. a. If yes for possession, place an X in the applicable box and if requested, include the quantity held in your lab. b. If no, leave blank. 2. After reviewing the list, please complete the information box below (or on last page for possession), then sign, date and return to EH&S. 3. Please call Donna Douglass at 275-2402 if you have any questions. Thank you for your cooperation in collecting data required by the Department of Homeland Security! Possession: 1) Fill in applicable boxes, 2) have PI sign last page, 3) return all pages to Donna Douglass OR Non-possession: 1) Check only one box on the left, 2) sign, 3) return just this page to Donna Douglass I do not have a lab, do not work in a lab, nor do I possess any of the agents in this survey. -

Oxygen - Wikipedia
5/20/2020 Oxygen - Wikipedia Oxygen Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in Oxygen, 8O the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. After hydrogen and helium, oxygen is the third-most abundant element in the universe by mass. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula O2. Diatomic oxygen gas constitutes 20.95% of the Earth's atmosphere. As compounds including oxides, the element makes up almost half of the Earth's crust.[2] Liquid oxygen boiling Dioxygen provides the energy released in combustion[3] and Oxygen aerobic cellular respiration,[4] and many major classes of Allotropes O2, O3 (ozone) organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats, as do Appearance gas: colorless the major constituent inorganic compounds of animal shells, liquid and solid: pale teeth, and bone. Most of the mass of living organisms is blue oxygen as a component of water, the major constituent of Standard atomic [15.999 03, 15.999 77] lifeforms. Oxygen is continuously replenished in Earth's weight A (O) conventional: 15.999 atmosphere by photosynthesis, which uses the energy of r, std sunlight to produce oxygen from water and carbon dioxide. Oxygen in the periodic table Oxygen is too chemically reactive to remain a free element in H H – air without being continuously replenished by the LB BCNOFN ↑ SM ASPSCA photosynthetic action of living organisms. -

1. Trioxygen Difluoride Reacts with Fluorine to Form A. Dioxygen Trifluoride B
1. Trioxygen difluoride reacts with fluorine to form a. Dioxygen trifluoride b. Dioxygen difluoride c. Oxygen difluoride d. Oxygen & fluorine 2. I2O4 and I2O9 are salt like and a. Are considered as iodine salt b. Are used in table salt c. Are called as iodine – iodates d. None of them 3. Reaction of HClO4 with P2O5 produces a. Chlorine heptaoxide b. Chlorine dioxide c. Dichlorine monoxide d. Chlorine hexaoxide 4. Reaction of bromine vapours with mercuric oxide yields a. Bromine dioxide b. Bromine trioxide c. Bromine monoxide d. All of them 5. Iodine pentaoxide acts as a. An oxidizing agent b. Reducing agent c. Bleaching agent d. Dehydrating agent 6. HClO2 is called a. Hypochlorous acid b. Chlorous acid c. Perchloric acid d. None of them 7. Which compound is used for the quantitative analysis of CO? a. O3F2 b. ClO2 c. Br2O d. I2O5 8. Bleaching powder can be manufactured by a. Hasenclever’s method b. Beckmann’s method c. Both a & b d. None of them 9. Bleaching powder oxidizes NH3 & yields a. Chlorine gas b. Nitrogen gas c. NH4Cl d. None of them 10. Freon is the commercial name of a. fluorochlorcarbons b. Tetrafluoroethylene c. Fluoroethylene d. None of them 11. Halothane is used as a. Disinfectant b. Anaesthetic c. Fungicicle d. Insecticide 12. Which one of the following halides has the highest lattlice energies? a. Iodides b. Chlorides c. Fluorides d. None of them 13. Which one of the following Halogens has the highest oxidizing power? a. F b. Cl c. I d. Br 14. Which one of the following Halogens can oxidize all other halide ions to molecular Halogen? a. -

Black Carbon and Its Impact on Earth's Climate
Lesson Plan: Black Carbon and its Impact on Earth’s Climate A teacher-contributed lesson plan by Dr. Shefali Shukla, Sri Venkateswara College (University of Delhi), India. As a High School or Undergraduate Chemistry or Environmental Sciences teacher, you can use this set of computer-based tools to teach about allotropy, various allotropes of carbon and their structural and physical properties, black carbon, sources of black carbon and its impact on Earth’s climate. This lesson plan will help students understand the concept of allotropy and various allotropes of carbons. Students will learn about black carbon, the effect of black carbon on the Earth’s albedo and therefore, its impact on the climate. This lesson plan will also help students to understand how the immediate effect of controlling black carbon emission can potentially slow down the rate of global warming. Thus, the use of this lesson plan allows you to integrate the teaching of a climate science topic with a core topic in Chemistry or Environmental Sciences. Use this lesson plan to help your students find answers to: • What are allotropes? What are the various allotropes of carbon and their properties? • What are the sources of black carbon? • What are the different effects of black carbon on clouds? How does it modify rainfall pattern? • How does the deposition of black carbon on ice caps affect melting of the ice? • Explain how black carbon can have a cooling or warming effect on the planet? • What is the effect of black carbon on human health? About the Lesson Plan Grade Level: High School, Undergraduate Discipline: Chemistry, Environmental Sciences Topic(s) in Discipline: Allotropy, Allotropes of carbon, Black Carbon, Sources of Black Carbon, Heating and Cooling Effects of Black Carbon, Effect of Black Carbon on Human Health, Black Carbon Albedo, Black Carbon Emission Climate Topic: Climate and the Atmosphere, The Greenhouse Gas Effect, Climate and the Anthroposphere Location: Global Access: Online, Offline Language(s): English Approximate Time Required: 90-120 min 1 Contents 1. -

Chemical Chemical Hazard and Compatibility Information
Chemical Chemical Hazard and Compatibility Information Acetic Acid HAZARDS & STORAGE: Corrosive and combustible liquid. Serious health hazard. Reacts with oxidizing and alkali materials. Keep above freezing point (62 degrees F) to avoid rupture of carboys and glass containers.. INCOMPATIBILITIES: 2-amino-ethanol, Acetaldehyde, Acetic anhydride, Acids, Alcohol, Amines, 2-Amino-ethanol, Ammonia, Ammonium nitrate, 5-Azidotetrazole, Bases, Bromine pentafluoride, Caustics (strong), Chlorosulfonic acid, Chromic Acid, Chromium trioxide, Chlorine trifluoride, Ethylene imine, Ethylene glycol, Ethylene diamine, Hydrogen cyanide, Hydrogen peroxide, Hydrogen sulfide, Hydroxyl compounds, Ketones, Nitric Acid, Oleum, Oxidizers (strong), P(OCN)3, Perchloric acid, Permanganates, Peroxides, Phenols, Phosphorus isocyanate, Phosphorus trichloride, Potassium hydroxide, Potassium permanganate, Potassium-tert-butoxide, Sodium hydroxide, Sodium peroxide, Sulfuric acid, n-Xylene. Acetone HAZARDS & STORAGE: Store in a cool, dry, well ventilated place. INCOMPATIBILITIES: Acids, Bromine trifluoride, Bromine, Bromoform, Carbon, Chloroform, Chromium oxide, Chromium trioxide, Chromyl chloride, Dioxygen difluoride, Fluorine oxide, Hydrogen peroxide, 2-Methyl-1,2-butadiene, NaOBr, Nitric acid, Nitrosyl chloride, Nitrosyl perchlorate, Nitryl perchlorate, NOCl, Oxidizing materials, Permonosulfuric acid, Peroxomonosulfuric acid, Potassium-tert-butoxide, Sulfur dichloride, Sulfuric acid, thio-Diglycol, Thiotrithiazyl perchlorate, Trichloromelamine, 2,4,6-Trichloro-1,3,5-triazine