Explain Kotar Classification
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

Guide to the Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- LILIACEAE
Guide to the Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- LILIACEAE LILIACEAE de Jussieu 1789 (Lily Family) (also see AGAVACEAE, ALLIACEAE, ALSTROEMERIACEAE, AMARYLLIDACEAE, ASPARAGACEAE, COLCHICACEAE, HEMEROCALLIDACEAE, HOSTACEAE, HYACINTHACEAE, HYPOXIDACEAE, MELANTHIACEAE, NARTHECIACEAE, RUSCACEAE, SMILACACEAE, THEMIDACEAE, TOFIELDIACEAE) As here interpreted narrowly, the Liliaceae constitutes about 11 genera and 550 species, of the Northern Hemisphere. There has been much recent investigation and re-interpretation of evidence regarding the upper-level taxonomy of the Liliales, with strong suggestions that the broad Liliaceae recognized by Cronquist (1981) is artificial and polyphyletic. Cronquist (1993) himself concurs, at least to a degree: "we still await a comprehensive reorganization of the lilies into several families more comparable to other recognized families of angiosperms." Dahlgren & Clifford (1982) and Dahlgren, Clifford, & Yeo (1985) synthesized an early phase in the modern revolution of monocot taxonomy. Since then, additional research, especially molecular (Duvall et al. 1993, Chase et al. 1993, Bogler & Simpson 1995, and many others), has strongly validated the general lines (and many details) of Dahlgren's arrangement. The most recent synthesis (Kubitzki 1998a) is followed as the basis for familial and generic taxonomy of the lilies and their relatives (see summary below). References: Angiosperm Phylogeny Group (1998, 2003); Tamura in Kubitzki (1998a). Our “liliaceous” genera (members of orders placed in the Lilianae) are therefore divided as shown below, largely following Kubitzki (1998a) and some more recent molecular analyses. ALISMATALES TOFIELDIACEAE: Pleea, Tofieldia. LILIALES ALSTROEMERIACEAE: Alstroemeria COLCHICACEAE: Colchicum, Uvularia. LILIACEAE: Clintonia, Erythronium, Lilium, Medeola, Prosartes, Streptopus, Tricyrtis, Tulipa. MELANTHIACEAE: Amianthium, Anticlea, Chamaelirium, Helonias, Melanthium, Schoenocaulon, Stenanthium, Veratrum, Toxicoscordion, Trillium, Xerophyllum, Zigadenus. -

ON the WOODY PLANTS of Llasca PARK a MAJOR REPORT
.. \ PATTERNS AND INTEI~ SITY OF WHI'l'E•TAILED DEER BROWSING ON THE WOODY PLANTS OF llASCA PARK ~ -·• .-:~~ -"7,"l!'~C .-'";"'l.;'3. • ..., 1; ;-:_ . .. ..... .. ;_., ( •• ~ -· ., : A MAJOR REPORT ~ i ..,; ·:-.:.. _, j i... ...... .. .' J £70 q ... c a:s·:, >rse·- _....,. •e '~· SUBMITTED TO THE.. SCHOOL OF FORESTRY By DONALD N. ORKE •' IN: PARTIAL FULFILU1ENT OF THE REQUIREMENTS. FOR THE DEGREE OF MASTER OF SCIENCE OCTOBER, 1966 - .. ,,..\ ., ..- ./ ACKNOWLEDGEMENTS The author wishes to thank the School of Forestry . :-~~ ..·,. for providing the, financial assistance needed to conduct the study. Also, special thf.llks ·are due to Dr. Henry L. Hansen who provided help:f'ul advice in th• preparation of. this report • .. - ' TABLE OF CONTENTS INTRODUCTION ••••••••••••••••••••••••••••••••••••••••• 1 HISTORY OF THE ITASCA DEER HERD AND ITS EFFECTS ON THE VEGETATION ••••••••••••••••••••••••••• •••••••••• 2 LITERATURE REVIEW •••••••••••••••••••••••••••••••••••• 5 STUDY AREA ••••••••••••••••••••••••••••••••••••••••• • 11 METH<D.DS:i •••• . .. .. .. .. .. .. .. .. .. .•• 13 RESULTS BROWSE PREFERENCES ••••••••••••••••••••••••••• 17 BROWSING ON KONIFER SEEDLINGS •••••••••••••••• 40 DISCUSSION BROWSE PREFERENCES ••••••••••••••••••••••••••• 51 BROWSING ON CONIFER SEEDLINGS •• • • • • • • • • • • • • • .54 SUGGESTIONS FOR FUTURE STUDIES ••••••••••••••• 56 SUMMA.RY • •••••••••••••••••••••••••••••••••••••••••••• 5 7 LITERATURE CITED ••••••••••••••••••••••••••••••• a.• .. 59 APPENDIX •••••••••••••••••••••• e ••••••••••••••••••••• 61 ,-·. INTRODUCTION -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Trillium, As an Indicator of Deer Density Hanover Biodiversity Committee October, 2017
[DRAFT v. 10] Trillium, as an indicator of deer density Hanover Biodiversity Committee October, 2017 Rationale for this Report Members of the lily family, such as Trillium and Clintonia, are among the favored foods of deer; 30 species of Trillium are found East of the Mississippi. The decline of these plants is mentioned in multiple publications1 as one key indicator of deer over-abundance. Red Trillium (Trillium erectum), also called ‘wake Robin’, found in the north-east and is (or was) fairly common in many Hanover forested neighborhoods. We suggest that monitoring this plant where it is (or once was) common demonstrates that deer density remains unsustainably high and future monitoring of the plant can help determine both the neighborhood density of deer and also serve as an indicator of change in deer density. Monitoring for this plant is easy, with just a small bit of training about the process. This report suggests a serious decline in biodiversity in Hanover over the past 15 years, as indicated by impact on red Trillium at three sites. We believe that with a focused increase in hunting pressure, this and other declining native plants might recover. Red Trillium is a frequent member of typical ‘rich mesic forests2’ plant communities found in Hanover; other plants often found nearby are Virginia waterleaf, blood root, wild ginger, foam flower, blue cohosh, and certain other members of the lily family. Besides aggressive deer browse, these communities are also threatened in varying degrees by invasive plants: garlic mustard, Dame’s rocket, wild parsnip, wild chervil and forget-me-not as well as the usual woody invaders. -
Species Lists
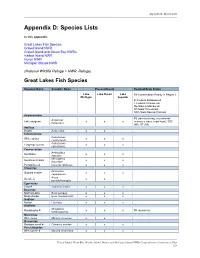
Appendix D: Species Lists Appendix D: Species Lists In this appendix: Great Lakes Fish Species Gravel Island NWR Gravel Island and Green Bay NWRs Harbor Island NWR Huron NWR Michigan Islands NWR (National Wildlife Refuge = NWR, Refuge) Great Lakes Fish Species Common Name Scientific Name Present/Absent Regional/State Status Lake Lake Huron Lake R3-Conservation Priority in Region 3 Michigan Superior E- Federal Endangered T-Federal Threatened SE-State Endangered ST-State Threatened SSC-State Special Concern Acipenseridae R3 (rare/declining, recreational/ Acipenser Lake sturgeon x x x economic value, tribal trust), SSC fulvescens (WI), ST (MI) Amiidae Bowfin Amia calva x x Catostomidae Catostomus White sucker x x x commersoni Catostomus Longnose sucker x x x catostomus Centrarchidae Ambloplites Rockbass x x x rupestris Micropterus Smallmouth bass x x x dolomieui Pumpkinseed Lepomis gibbosus x x x Clupeidae Dorosoma Gizzard shad # x x x cepedianum Alosa Alewife # x x pseudoharengus Cyprinidae Carp # Cyprinus Carpio x x x Esocidae Northern pike Esox Lucieus x x x Muskellunge Esox masquinongy x x x Gadidae Burbot Lota lota x x x Gobiidae Neogobius Round goby # x x x R3 (nuisance) melanostomus Moronidae White bass Morone chrysops x x Osmeridae Rainbow smelt # Osmerus mordax x x x Percichthyidae White perch # Morone americana x x x Gravel Island, Green Bay, Harbor Island, Huron, and Michigan Islands NWRs/Comprehensive Conservation Plan 221 Appendix D: Species Lists Common Name Scientific Name Present/Absent Regional/State Status Percidae R3 (rare/declining, -

South Carolina Wildflowers by Color and Season
SOUTH CAROLINA WILDFLOWERS *Chokeberry (Aronia arbutifolia) Silky Camellia (Stewartia malacodendron) BY COLOR AND SEASON Mountain Camelia (Stewartia ovata) Dwarf Witch Alder (Fothergilla gardenii) Revised 10/2007 by Mike Creel *Wild Plums (Prunus angustifolia, americana) 155 Cannon Trail Road Flatwoods Plum (Prunus umbellata) Lexington, SC 29073 *Shadberry or Sarvis Tree (Amelanchier arborea, obovata) Phone: (803) 359-2717 E-mail: [email protected] Fringe Tree (Chionanthus virginicus) Yellowwood Tree (Cladratis kentuckeana) Silverbell Tree (Halesia carolina, etc.) IDENTIFY PLANTS BY COLOR, THEN Evergreen Cherry Laurel (Prunus caroliniana) SEASON . Common ones in bold print. Hawthorn (Crataegus viridis, marshalli, etc.) Storax (Styrax americana, grandifolia) Wild Crabapple (Malus angustifolia) WHITE Wild Cherry (Prunus serotina) SPRING WHITE Dec. 1 to May 15 SUMMER WHITE May 15 to Aug. 7 *Atamasco Lily (Zephyranthes atamasco) *Swamp Spiderlily (Hymenocallis crassifolia) Carolina Anemone (Anemone caroliniana) Rocky Shoals Spiderlily (Hymenocallis coronaria) Lance-leaved Anemone (Anemone lancifolia) Colic Root (Aletris farinosa) Meadow Anemone (Anemone canadensis) Fly-Poison (Amianthium muscaetoxicum) American Wood Anemone (Anemone quinquefolia) Angelica (Angelica venosa) Wild Indigo (Baptisia bracteata) Ground Nut Vine (Apios americana) Sandwort (Arenaria caroliniana) Indian Hemp (Apocynum cannabium) American Bugbane (Cimicifuga americana) Sand Milkweed (Asclepias humistrata) Cohosh Bugbane (Cimicifuga racemosa) White Milkweed (Asclepias -

Illinois Exotic Species List
Exotic Species in Illinois Descriptions for these exotic species in Illinois will be added to the Web page as time allows for their development. A name followed by an asterisk (*) indicates that a description for that species can currently be found on the Web site. This list does not currently name all of the exotic species in the state, but it does show many of them. It will be updated regularly with additional information. Microbes viral hemorrhagic septicemia Novirhabdovirus sp. West Nile virus Flavivirus sp. Zika virus Flavivirus sp. Fungi oak wilt Ceratocystis fagacearum chestnut blight Cryphonectria parasitica Dutch elm disease Ophiostoma novo-ulmi and Ophiostoma ulmi late blight Phytophthora infestans white-nose syndrome Pseudogymnoascus destructans butternut canker Sirococcus clavigignenti-juglandacearum Plants okra Abelmoschus esculentus velvet-leaf Abutilon theophrastii Amur maple* Acer ginnala Norway maple Acer platanoides sycamore maple Acer pseudoplatanus common yarrow* Achillea millefolium Japanese chaff flower Achyranthes japonica Russian knapweed Acroptilon repens climbing fumitory Adlumia fungosa jointed goat grass Aegilops cylindrica goutweed Aegopodium podagraria horse chestnut Aesculus hippocastanum fool’s parsley Aethusa cynapium crested wheat grass Agropyron cristatum wheat grass Agropyron desertorum corn cockle Agrostemma githago Rhode Island bent grass Agrostis capillaris tree-of-heaven* Ailanthus altissima slender hairgrass Aira caryophyllaea Geneva bugleweed Ajuga genevensis carpet bugleweed* Ajuga reptans mimosa -

Appendix 2: Plant Lists
Appendix 2: Plant Lists Master List and Section Lists Mahlon Dickerson Reservation Botanical Survey and Stewardship Assessment Wild Ridge Plants, LLC 2015 2015 MASTER PLANT LIST MAHLON DICKERSON RESERVATION SCIENTIFIC NAME NATIVENESS S-RANK CC PLANT HABIT # OF SECTIONS Acalypha rhomboidea Native 1 Forb 9 Acer palmatum Invasive 0 Tree 1 Acer pensylvanicum Native 7 Tree 2 Acer platanoides Invasive 0 Tree 4 Acer rubrum Native 3 Tree 27 Acer saccharum Native 5 Tree 24 Achillea millefolium Native 0 Forb 18 Acorus calamus Alien 0 Forb 1 Actaea pachypoda Native 5 Forb 10 Adiantum pedatum Native 7 Fern 7 Ageratina altissima v. altissima Native 3 Forb 23 Agrimonia gryposepala Native 4 Forb 4 Agrostis canina Alien 0 Graminoid 2 Agrostis gigantea Alien 0 Graminoid 8 Agrostis hyemalis Native 2 Graminoid 3 Agrostis perennans Native 5 Graminoid 18 Agrostis stolonifera Invasive 0 Graminoid 3 Ailanthus altissima Invasive 0 Tree 8 Ajuga reptans Invasive 0 Forb 3 Alisma subcordatum Native 3 Forb 3 Alliaria petiolata Invasive 0 Forb 17 Allium tricoccum Native 8 Forb 3 Allium vineale Alien 0 Forb 2 Alnus incana ssp rugosa Native 6 Shrub 5 Alnus serrulata Native 4 Shrub 3 Ambrosia artemisiifolia Native 0 Forb 14 Amelanchier arborea Native 7 Tree 26 Amphicarpaea bracteata Native 4 Vine, herbaceous 18 2015 MASTER PLANT LIST MAHLON DICKERSON RESERVATION SCIENTIFIC NAME NATIVENESS S-RANK CC PLANT HABIT # OF SECTIONS Anagallis arvensis Alien 0 Forb 4 Anaphalis margaritacea Native 2 Forb 3 Andropogon gerardii Native 4 Graminoid 1 Andropogon virginicus Native 2 Graminoid 1 Anemone americana Native 9 Forb 6 Anemone quinquefolia Native 7 Forb 13 Anemone virginiana Native 4 Forb 5 Antennaria neglecta Native 2 Forb 2 Antennaria neodioica ssp. -

Phylogeny and Phylogenetic Taxonomy of Dipsacales, with Special Reference to Sinadoxa and Tetradoxa (Adoxaceae)
PHYLOGENY AND PHYLOGENETIC TAXONOMY OF DIPSACALES, WITH SPECIAL REFERENCE TO SINADOXA AND TETRADOXA (ADOXACEAE) MICHAEL J. DONOGHUE,1 TORSTEN ERIKSSON,2 PATRICK A. REEVES,3 AND RICHARD G. OLMSTEAD 3 Abstract. To further clarify phylogenetic relationships within Dipsacales,we analyzed new and previously pub- lished rbcL sequences, alone and in combination with morphological data. We also examined relationships within Adoxaceae using rbcL and nuclear ribosomal internal transcribed spacer (ITS) sequences. We conclude from these analyses that Dipsacales comprise two major lineages:Adoxaceae and Caprifoliaceae (sensu Judd et al.,1994), which both contain elements of traditional Caprifoliaceae.Within Adoxaceae, the following relation- ships are strongly supported: (Viburnum (Sambucus (Sinadoxa (Tetradoxa, Adoxa)))). Combined analyses of C ap ri foliaceae yield the fo l l ow i n g : ( C ap ri folieae (Diervilleae (Linnaeeae (Morinaceae (Dipsacaceae (Triplostegia,Valerianaceae)))))). On the basis of these results we provide phylogenetic definitions for the names of several major clades. Within Adoxaceae, Adoxina refers to the clade including Sinadoxa, Tetradoxa, and Adoxa.This lineage is marked by herbaceous habit, reduction in the number of perianth parts,nectaries of mul- ticellular hairs on the perianth,and bifid stamens. The clade including Morinaceae,Valerianaceae, Triplostegia, and Dipsacaceae is here named Valerina. Probable synapomorphies include herbaceousness,presence of an epi- calyx (lost or modified in Valerianaceae), reduced endosperm,and distinctive chemistry, including production of monoterpenoids. The clade containing Valerina plus Linnaeeae we name Linnina. This lineage is distinguished by reduction to four (or fewer) stamens, by abortion of two of the three carpels,and possibly by supernumerary inflorescences bracts. Keywords: Adoxaceae, Caprifoliaceae, Dipsacales, ITS, morphological characters, phylogeny, phylogenetic taxonomy, phylogenetic nomenclature, rbcL, Sinadoxa, Tetradoxa. -

(Betula Papyrifera) / Diervilla Lonicera Trembling Aspen (Paper Birch) / Northern Bush-Honeysuckle Peuplier Faux-Tremble (Bouleau À Papier) / Dièreville Chèvrefeuille
Forest / Forêt Association CNVC 00238 Populus tremuloides (Betula papyrifera) / Diervilla lonicera Trembling Aspen (Paper Birch) / Northern Bush-honeysuckle Peuplier faux-tremble (Bouleau à papier) / Dièreville chèvrefeuille Subassociations: 238a typic, 238b Alnus viridis, 238c Kalmia angustifolia CNVC Alliance: CA00014 Betula papyrifera – Populus tremuloides – Abies balsamea / Clintonia borealis CNVC Group: CG0007 Ontario-Quebec Boreal Mesic Paper Birch – Balsam Fir – Trembling Aspen Forest Type Description Concept: CNVC00238 is a boreal hardwood forest Association that ranges from Manitoba to Quebec. It has a closed canopy dominated by trembling aspen ( Populus tremuloides ), usually with paper birch ( Betula papyrifera ), overtopping a well-developed to dense shrub layer. The shrub layer includes a mix of regenerating tree species, primarily trembling aspen, balsam fir ( Abies balsamea ), paper birch and black spruce ( Picea mariana ), as well as low shrub species, such as velvet-leaved blueberry ( Vaccinium myrtilloides ), northern bush-honeysuckle ( Diervilla lonicera ) and early lowbush blueberry ( V. angustifolium ). The herb layer is well developed and typically includes bunchberry (Cornus canadensis ), wild lily-of-the-valley ( Maianthemum canadense ), wild sarsaparilla ( Aralia nudicaulis ), yellow clintonia ( Clintonia borealis ), twinflower ( Linnaea borealis ) and northern starflower Source: Natural Resources Canada - (Lysimachia borealis ). The forest floor cover is mainly broad-leaf litter so the moss layer is Canadian Forest Service sparse, with only minor cover of red-stemmed feathermoss ( Pleurozium schreberi) . CNVC00238 is an early seral condition that typically establishes after fire or harvesting. It occurs in a region with a continental boreal climate that grades from subhumid in the west to humid in the east and is usually found on mesic, nutrient-medium sites. -

Liliaceae Lily Family
Liliaceae lily family While there is much compelling evidence available to divide this polyphyletic family into as many as 25 families, the older classification sensu Cronquist is retained here. Page | 1222 Many are familiar as garden ornamentals and food plants such as onion, garlic, tulip and lily. The flowers are showy and mostly regular, three-merous and with a superior ovary. Key to genera A. Leaves mostly basal. B B. Flowers orange; 8–11cm long. Hemerocallis bb. Flowers not orange, much smaller. C C. Flowers solitary. Erythronium cc. Flowers several to many. D D. Leaves linear, or, absent at flowering time. E E. Flowers in an umbel, terminal, numerous; leaves Allium absent. ee. Flowers in an open cluster, or dense raceme. F F. Leaves with white stripe on midrib; flowers Ornithogalum white, 2–8 on long peduncles. ff. Leaves green; flowers greenish, in dense Triantha racemes on very short peduncles. dd. Leaves oval to elliptic, present at flowering. G G. Flowers in an umbel, 3–6, yellow. Clintonia gg. Flowers in a one-sided raceme, white. Convallaria aa. Leaves mostly cauline. H H. Leaves in one or more whorls. I I. Leaves in numerous whorls; flowers >4cm in diameter. Lilium ii. Leaves in 1–2 whorls; flowers much smaller. J J. Leaves 3 in a single whorl; flowers white or purple. Trillium jj. Leaves in 2 whorls, or 5–9 leaves; flowers yellow, small. Medeola hh. Leaves alternate. K K. Flowers numerous in a terminal inflorescence. L L. Plants delicate, glabrous; leaves 1–2 petiolate. Maianthemum ll. Plant coarse, robust; stems pubescent; leaves many, clasping Veratrum stem.