The Limited Diverence Between Keratin Patterns of Squamous Cell
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Keratin 19 Regulates Cell Shape and Cell-Cell Adhesion of MCF7 Cells While Maintaining E-Cadherin Localization at the Cell Surface
Keratin 19 regulates cell shape and cell-cell adhesion of MCF7 cells while maintaining E-cadherin localization at the cell surface Welcome to my poster. This is Sarah Alsharif, a PhD student from the biology department. I am glad to present the work our lab has been doing in the breast cancer field. In fact, after lung cancer, breast cancer is the second cause of death in women worldwide (1). It is estimated that every 18 seconds, approximately one new case of breast cancer is documented (2). No one dies due to cancer itself. The death is because of metastasis which takes place when cancer cells leave a breast in which they are formed and reach other sites such as the brain or lung. Our lab is interested in investigating the mechanism behind metastasis of breast cancer. Metastasis is associated with what is called epithelial to mesenchymal transition (EMT), the process characterized by loss of cell to cell adhesion and expression of epithelial markers such as keratin intermediate filament proteins, as you can see in the first three images of cells. Those filaments are keratins and they are critical for the shape and for maintaining mechanical integrity of epithelial cells via cell to cell complexes called desmosomes. Among different keratins, keratin 19 (K19) is highly expressed in many types of cancer including breast cancer, and is correlated with a worse prognosis (3). Consistently, K19 expression has been reported to be significantly higher in metastatic breast cancer tumor cells compared to primary tumors (4). The role of K19 on mechanical properties of cancer cells for cell migration and possible impact on metastasis in breast cancer patients is still unknown. -

In Situlocalization of Cytoskeletal Elements in the Human Trabecular
Investigative Ophthalmology & Visual Science. Vol. 31. No. 9. September 1990 Cops right £• Association lor Research in Vision and Ophthalmology In Situ Localization of Cytoskeletal Elements in the Human Trabecular Meshwork and Cornea Robert N. Weinreb* and Mark I. Ryderf The authors compared cytoskeletal elements of the in situ human trabccular-mcshwork cell with in situ human corneal cells using indirect immunofluorcsccncc staining for tubulin and intermediate filaments (vimentin, cytokeratin, and desmin) and NBD-phallacidin staining for f-actin using both fixed frozen and unfixed frozen sections from postmortem eyes. Both f-actin and tubulin were found throughout the cell body of trabecular-meshwork cells, keratocytes, corneal endothelium, and corneal epithelium. The f-actin staining pattern was concentrated at the cell periphery of these four cell types. Vimentin stain was intensely localized in focal areas of the trabecular-meshwork cell, keratocytes, and throughout the corneal cndothelium. A general anticytokeratin antibody was intensely localized in corneal epithelium and endothelium. However, PKK-1 anticytokeratin antibody was seen only in superficial layers of corneal epithelium and not in corneal endothelium. The 4.62 anticytokeratin antibody was not observed in either corneal epithelium or endothelium. None of these three cytokera- tin antibodies were seen in trabccular-mcshwork cells or keratocytes. Desmin stain was not noted in any of these cell types. In general, cytoskeletal staining of unfixed frozen sections showed a similar staining pattern for f-actin and tubulin but a more uniform and intense staining pattern for vimentin and cytokcratin compared with fixed frozen material. The authors conclude that these cytoskclctal stains can differentiate human Irabeciilar-meshwork cells from cells of the cornea in situ. -

Inhibiting TNIK for Treating Colon Cancer
(19) & (11) EP 2 305 717 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 06.04.2011 Bulletin 2011/14 C07K 16/40 (2006.01) C12N 15/11 (2006.01) C12Q 1/48 (2006.01) C12Q 1/68 (2006.01) (2006.01) (21) Application number: 09170853.7 G01N 33/50 (22) Date of filing: 21.09.2009 (84) Designated Contracting States: • Mahmoudi, Tokameh AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 3515 XS, Utrecht (NL) HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL • Clevers, Johannes Carolus PT RO SE SI SK SM TR 3712 AP, Huis ter Heide (NL) (71) Applicant: KoninklijkeNederlandse Akademie van (74) Representative: Swinkels, Bart Willem Wetenschappen Nederlandsch Octrooibureau 1011 JV Amsterdam (NL) J. W. Frisolaan 13 2517 JS Den Haag (NL) (72) Inventors: • Wing Li, Vivian Sze 3572 SH, Utrecht (NL) (54) Inhibiting TNIK for treating colon cancer (57) The invention relates to an inhibitor of TNIK and its use for treating cancer. EP 2 305 717 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 305 717 A1 Description Field of the invention 5 [0001] The invention relates to an inhibitor of TNIK and its use as a medicament for treating cancer. Background of the invention [0002] The primary function of the intestinal tract involves the digestion and absorption of nutrients. The intestinal 10 lumen is lined with a specialized simple epithelium, which performs the primary functions of digestion, water and nutrient absorption and forms a barrier against luminal pathogens. -

Decreased Expression of Profilin 2 in Oral Squamous Cell Carcinoma and Its Clinicopathological Implications
ONCOLOGY REPORTS 26: 813-823, 2011 Decreased expression of profilin 2 in oral squamous cell carcinoma and its clinicopathological implications C.Y. MA1,2, C.P. ZHANG1,2, L.P. ZHONG1,2, H.Y. PAN1,2, W.T. CHEN1,2, L.Z. WANG3, O.W. ANDREW4, T. JI1 and W. HAN1,2 1Department of Oral and Maxillofacial Surgery, Ninth People's Hospital, College of Stomatology; 2Shanghai Key Laboratory of Stomatology and Shanghai Research Institute of Stomatology; 3Department of Oral Pathology, Ninth People's Hospital, College of Stomatology, Shanghai Jiao Tong University School of Medicine, Shanghai 200011, P.R. China; 4Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, National University of Singapore, Singapore 119074, Singapore Received February 8, 2011; Accepted April 11, 2011 DOI: 10.3892/or.2011.1365 Abstract. Profilins are small proteins essential for many clinical and pathological significance. In conclusion, PFN2 normal cellular dynamics and constitute one of the crucial can be utilized as both a potential suppressor marker and a components of actin-based cellular motility. Several recent prognostic protein for OSCC. The function of PFN2 may be to studies have implicated a role for the profilin (PFN) family in regulate the N-WASP/Arp2/3 signaling pathway. cancer pathogenesis and progression. However, their expression and promising functions are largely unknown in oral squamous Introduction cell carcinoma (OSCC). In this study, we analyzed the correlation between PFN1 and PFN2 expression in vitro and Oral squamous cell carcinoma (OSCC) is a significant public in vivo. The protein expression levels were roughly compared health problem with >300,000 new cases being diagnosed between cell lines (HIOEC, HB96) with the employment of annually worldwide (1). -

Genetic Background Effects of Keratin 8 and 18 in a DDC-Induced Hepatotoxicity and Mallory-Denk Body Formation Mouse Model
Laboratory Investigation (2012) 92, 857–867 & 2012 USCAP, Inc All rights reserved 0023-6837/12 $32.00 Genetic background effects of keratin 8 and 18 in a DDC-induced hepatotoxicity and Mallory-Denk body formation mouse model Johannes Haybaeck1, Cornelia Stumptner1, Andrea Thueringer1, Thomas Kolbe2, Thomas M Magin3, Michael Hesse4, Peter Fickert5, Oleksiy Tsybrovskyy1, Heimo Mu¨ller1, Michael Trauner5,6, Kurt Zatloukal1 and Helmut Denk1 Keratin 8 (K8) and keratin 18 (K18) form the major hepatocyte cytoskeleton. We investigated the impact of genetic loss of either K8 or K18 on liver homeostasis under toxic stress with the hypothesis that K8 and K18 exert different functions. krt8À/À and krt18À/À mice crossed into the same 129-ola genetic background were treated by acute and chronic ad- ministration of 3,5-diethoxy-carbonyl-1,4-dihydrocollidine (DDC). In acutely DDC-intoxicated mice, macrovesicular steatosis was more pronounced in krt8À/À and krt18À/À compared with wild-type (wt) animals. Mallory-Denk bodies (MDBs) appeared in krt18À/À mice already at an early stage of intoxication in contrast to krt8À/À mice that did not display MDB formation when fed with DDC. Keratin-deficient mice displayed significantly lower numbers of apoptotic hepatocytes than wt animals. krt8À/À, krt18À/À and control mice displayed comparable cell proliferation rates. Chronically DDC-intoxicated krt18À/À and wt mice showed a similarly increased degree of steatohepatitis with hepatocyte ballooning and MDB formation. In krt8À/À mice, steatosis was less, ballooning, and MDBs were absent. krt18À/À mice developed MDBs whereas krt8À/À mice on the same genetic background did not, highlighting the significance of different structural properties of keratins. -

Study for Pathogenesis of Congenital Cholesteatoma with Comparison of Proteins Expressed in Congenital Cholesteatoma, Acquired C
Study for pathogenesis of congenital cholesteatoma with comparison of proteins expressed in congenital cholesteatoma, acquired cholesteatoma and skin of the external auditory canal through proteomics Seung Ho Shin Department of Medicine The Graduate School, Yonsei University Study for pathogenesis of congenital cholesteatoma with comparison of proteins expressed in congenital cholesteatoma, acquired cholesteatoma and skin of the external auditory canal through proteomics Directed by Professor Jae Young Choi The Doctoral Dissertation submitted to the Department of Medicine, the Graduate School of Yonsei University in partial fulfillment of the requirements for the degree of Doctor of Philosophy Seung Ho Shin June 2014 ACKNOWLEDGEMENTS In the initial period of my fellowship, I wrote a book, Temporal Bone Dissection Manual as a coauthor with Professor Won Sang Lee, Ho-Ki Lee and Jae Young Choi. Through this book, I learned about much knowledge from them. Professor Jae Young Choi advised me to go for a Ph.D. I admitted graduate school for a Ph.D. in 2007. In my doctoral course, he has instructed me in detail on the basic research. He has demonstrated precise and delicate laboratory techniques and showed outstanding ability to create new ideas. Also, he has often said to me that a researcher must be honest to his colleagues and even to himself. After the summer of 2013, he gave me an idea for this paper, which was for congenital cholesteatoma analysis with proteomics. He always displayed endless energy and enthusiasm for scientific experiments even after many demanding surgeries. His passion motivated me to follow suit and seven months of our work at last bore fruit. -
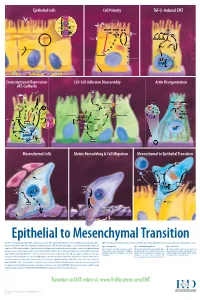
Epithelial to Mesenchymal Transition
Epithelial Cells Cell Polarity TGF-b-Induced EMT MUC-1 O-glycosylation Epithelial Cells ZO-1 Occludin Apical Membrane Tight F-Actin Microvilli Junction Claudin F-Actin p120 β-Catenin Adherens F-Actin Ezrin TGF-β dimer Junction E-Cadherin α-Catenin Plakophilin Crumbs Complex PAR Complex Desmocollin Desmoplakin Desmosome PtdIns(4,5)P2 TGF-β RII TGF-β RI CRB Cdc42Par6 Desmoglein Cytokeratin Pals1 PatJ Tight Junction Plakoglobin aPKC Par3 Domain Smad7 Extracellular PTEN JNK ERK1/2 p38 SARA Smurf1 Cortical Actin Cytoskeleton Space Par3 ZO-1 Adherens Junction PI 3-K Domain Smad-independent Signaling (–) Smad7 Translocation Smad2/3 PtdIns(3,4,5)P3 Smad4 Smad4 NEDD4 Cytokeratin Intermediate Filaments Smad2 Smad4 Smad3 LLGL Proteasome SCRIB DLG Scribble Complex Fibronectin Twist Smad2/3 Vitronectin ZEB 1/2 Microtubule Network Smad4 N-Cadherin Snail Basolateral Membrane CoA, Collagen I Slug CoR MMPs DNA-binding (+) Claudin Desmoplakin Transcription Factor Occludin Cytokeratins E-Cadherin Plakoglobin Integrins β α Nidogen-1/Entactin Perlecan Laminin Collagen IV Transcriptional Repression Cell-Cell Adhesion Disassembly Actin Reorganization of E-Cadherin TGF-β dimer EGF TGF-β RII TGF-β RI IGF FGF Receptor TNF-α Tyrosine Kinase Par6 TNF RI Apical Focal Adhesion Constriction Actin Depolymerization F-Actin Smurf1 Occludin Wnt Frizzled Myosin II Ras RhoA α-Actinin Myosin II ROCK AxinCK1 Dishevelled GSK-3 PI 3-K Src Zyxin MLC Phosphatase APC Proteasome FAK Vinculin RhoA ILK Talin (Inactive) Hakai Talin FAK F-Actin E-Cadherin LIMK Akt Paxillin FAK Stress -

Molecular Pathways Associated with the Nutritional Programming of Plant-Based Diet Acceptance in Rainbow Trout Following an Early Feeding Exposure Mukundh N
Molecular pathways associated with the nutritional programming of plant-based diet acceptance in rainbow trout following an early feeding exposure Mukundh N. Balasubramanian, Stéphane Panserat, Mathilde Dupont-Nivet, Edwige Quillet, Jérôme Montfort, Aurélie Le Cam, Françoise Médale, Sadasivam J. Kaushik, Inge Geurden To cite this version: Mukundh N. Balasubramanian, Stéphane Panserat, Mathilde Dupont-Nivet, Edwige Quillet, Jérôme Montfort, et al.. Molecular pathways associated with the nutritional programming of plant-based diet acceptance in rainbow trout following an early feeding exposure. BMC Genomics, BioMed Central, 2016, 17 (1), pp.1-20. 10.1186/s12864-016-2804-1. hal-01346912 HAL Id: hal-01346912 https://hal.archives-ouvertes.fr/hal-01346912 Submitted on 19 Jul 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Balasubramanian et al. BMC Genomics (2016) 17:449 DOI 10.1186/s12864-016-2804-1 RESEARCH ARTICLE Open Access Molecular pathways associated with the nutritional programming of plant-based diet acceptance in rainbow trout following an early feeding exposure Mukundh N. Balasubramanian1, Stephane Panserat1, Mathilde Dupont-Nivet2, Edwige Quillet2, Jerome Montfort3, Aurelie Le Cam3, Francoise Medale1, Sadasivam J. Kaushik1 and Inge Geurden1* Abstract Background: The achievement of sustainable feeding practices in aquaculture by reducing the reliance on wild-captured fish, via replacement of fish-based feed with plant-based feed, is impeded by the poor growth response seen in fish fed high levels of plant ingredients. -
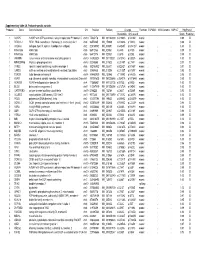
Supplementary Table 2A. Proband-Specific Variants Proband
Supplementary table 2A. Proband-specific variants Proband Gene Gene full name Chr Position RefSeqChange Function ESP6500 1000 Genome SNP137 PolyPhen2 Nucleotide Amino acid Score Prediction 1 AGAP5 ArfGAP with GTPase domain, ankyrin repeat and PH domain 5 chr10 75435178 NM_001144000 c.G1240A p.V414M exonic . 0.99 D 1 REXO1L1 REX1, RNA exonuclease 1 homolog (S. cerevisiae)-like 1 chr8 86574465 NM_172239 c.A1262G p.Y421C exonic . 0.98 D 1 COL4A3 collagen, type IV, alpha 3 (Goodpasture antigen) chr2 228169782 NM_000091 c.G4235T p.G1412V exonic . 1.00 D 1 KIAA1586 KIAA1586 chr6 56912133 NM_020931 c.C44G p.A15G exonic . 0.99 D 1 KIAA1586 KIAA1586 chr6 56912176 NM_020931 c.C87G p.D29E exonic . 0.99 D 1 JAKMIP3 Janus kinase and microtubule interacting protein 3 chr10 133955524 NM_001105521 c.G1574C p.G525A exonic . 1.00 D 1 MPHOSPH8 M-phase phosphoprotein 8 chr13 20240685 NM_017520 c.C2140T p.L714F exonic . 0.97 D 1 SYNE1 spectrin repeat containing, nuclear envelope 1 chr6 152783902 NM_033071 c.A2242T p.N748Y exonic . 0.97 D 1 CAND2 cullin-associated and neddylation-dissociated 2 (putative) chr3 12858835 NM_012298 c.C2125T p.H709Y exonic . 0.95 D 1 TDRD9 tudor domain containing 9 chr14 104460909 NM_153046 c.T1289G p.V430G exonic . 0.96 D 2 ASPM asp (abnormal spindle) homolog, microcephaly associated (Drosophila)chr1 197091625 NM_001206846 c.G3491A p.R1164H exonic . 0.99 D 2 ADAM29 ADAM metallopeptidase domain 29 chr4 175896951 NM_001130705 c.A275G p.D92G exonic . 1.00 D 2 BCO2 beta-carotene oxygenase 2 chr11 112087019 NM_001256398 c.G1373A p.G458E exonic . -

CDH12 Cadherin 12, Type 2 N-Cadherin 2 RPL5 Ribosomal
5 6 6 5 . 4 2 1 1 1 2 4 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 2 2 A A A A A A A A A A A A A A A A A A A A C C C C C C C C C C C C C C C C C C C C R R R R R R R R R R R R R R R R R R R R B , B B B B B B B B B B B B B B B B B B B , 9 , , , , 4 , , 3 0 , , , , , , , , 6 2 , , 5 , 0 8 6 4 , 7 5 7 0 2 8 9 1 3 3 3 1 1 7 5 0 4 1 4 0 7 1 0 2 0 6 7 8 0 2 5 7 8 0 3 8 5 4 9 0 1 0 8 8 3 5 6 7 4 7 9 5 2 1 1 8 2 2 1 7 9 6 2 1 7 1 1 0 4 5 3 5 8 9 1 0 0 4 2 5 0 8 1 4 1 6 9 0 0 6 3 6 9 1 0 9 0 3 8 1 3 5 6 3 6 0 4 2 6 1 0 1 2 1 9 9 7 9 5 7 1 5 8 9 8 8 2 1 9 9 1 1 1 9 6 9 8 9 7 8 4 5 8 8 6 4 8 1 1 2 8 6 2 7 9 8 3 5 4 3 2 1 7 9 5 3 1 3 2 1 2 9 5 1 1 1 1 1 1 5 9 5 3 2 6 3 4 1 3 1 1 4 1 4 1 7 1 3 4 3 2 7 6 4 2 7 2 1 2 1 5 1 6 3 5 6 1 3 6 4 7 1 6 5 1 1 4 1 6 1 7 6 4 7 e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e e l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p p m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m m -

Spiny Mice (Acomys) Exhibit Attenuated Hallmarks of Aging And
bioRxiv preprint doi: https://doi.org/10.1101/2020.05.07.083287; this version posted May 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 2 3 4 5 6 Title: Spiny mice (Acomys) exhibit attenuated hallmarks of aging and rapid cell turnover after UV 7 exposure in the skin epidermis 8 Authors: Wesley Wong1, Austin Kim1, Ashley W. Seifert2, Malcolm Maden3, and Justin D. Crane1 9 Affiliations: 1Department of Biology, Northeastern University, 360 Huntington Avenue, Boston, 10 MA 02115, 2Department of Biology, University of Kentucky, Lexington, KY 40506, 3UF 11 Genetics Institute & Department of Biology, University of Florida, Gainesville, FL 32611 12 13 Keywords: Spiny mouse, skin, epidermis, UV radiation, aging 14 15 16 17 18 19 20 21 22 23 24 25 26 27 bioRxiv preprint doi: https://doi.org/10.1101/2020.05.07.083287; this version posted May 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 28 Abstract 29 The study of long-lived and regenerative animal models has revealed diverse protective responses 30 to stressors such as aging and tissue injury. Spiny mice (Acomys) are a unique mammalian model 31 of skin regeneration, but their response to other types of physiological skin damage have not been 32 investigated. -

Quantigene Flowrna Probe Sets Currently Available
QuantiGene FlowRNA Probe Sets Currently Available Accession No. Species Symbol Gene Name Catalog No. NM_003452 Human ZNF189 zinc finger protein 189 VA1-10009 NM_000057 Human BLM Bloom syndrome VA1-10010 NM_005269 Human GLI glioma-associated oncogene homolog (zinc finger protein) VA1-10011 NM_002614 Human PDZK1 PDZ domain containing 1 VA1-10015 NM_003225 Human TFF1 Trefoil factor 1 (breast cancer, estrogen-inducible sequence expressed in) VA1-10016 NM_002276 Human KRT19 keratin 19 VA1-10022 NM_002659 Human PLAUR plasminogen activator, urokinase receptor VA1-10025 NM_017669 Human ERCC6L excision repair cross-complementing rodent repair deficiency, complementation group 6-like VA1-10029 NM_017699 Human SIDT1 SID1 transmembrane family, member 1 VA1-10032 NM_000077 Human CDKN2A cyclin-dependent kinase inhibitor 2A (melanoma, p16, inhibits CDK4) VA1-10040 NM_003150 Human STAT3 signal transducer and activator of transcripton 3 (acute-phase response factor) VA1-10046 NM_004707 Human ATG12 ATG12 autophagy related 12 homolog (S. cerevisiae) VA1-10047 NM_000737 Human CGB chorionic gonadotropin, beta polypeptide VA1-10048 NM_001017420 Human ESCO2 establishment of cohesion 1 homolog 2 (S. cerevisiae) VA1-10050 NM_197978 Human HEMGN hemogen VA1-10051 NM_001738 Human CA1 Carbonic anhydrase I VA1-10052 NM_000184 Human HBG2 Hemoglobin, gamma G VA1-10053 NM_005330 Human HBE1 Hemoglobin, epsilon 1 VA1-10054 NR_003367 Human PVT1 Pvt1 oncogene homolog (mouse) VA1-10061 NM_000454 Human SOD1 Superoxide dismutase 1, soluble (amyotrophic lateral sclerosis 1 (adult))