Basic Anatomy of an Oyster
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Branding for Sabah Pearl 2018
BRANDING FOR SABAH PEARL Tan Yin Yin Bachelor of Applied Arts with Honours (Design Technology) 2018 BRANDII\'G FOR SABAH PEARL 'IlL'1 YII\' YIN Th,s project is submitted in partial fulfillment of the requirements for the degree of Bachelor of Arts with Honors (IlE'sign Technology) Faculty of Apphed and Creative Arts UWVERSITI NiALl, YSIA SARAWAK 2013 PENJENAMAAN UNTUK MUTIARA SABAH TAN \1N \1N Pr'Diekilll merupakan salah satu keperluan untuk ljazah Sarjana Seni Gunaan dengan (Teknologi Sem Reka) Fakulti Seni. Gunaan dan Kreatif UNlVERSITI Mll.Lll. YSIA SARA WAK 2018 ii PIE'.u~ (leI> ( 'd FUll.! \'t","\r ?r -:~ <:' 1 R~pc.n :J :·::: ~r s PhD O(C'L\.R:HIO:--: Of ORIGI);_\L \,"ORb. dar ot ._ ;':II I S .Studt-nt's Decl"l'.1(1oD r . ICI"YtYl)!h (J 1f O. ~7.)~m fa(,tlt'r° fAr p.ii~4~f). (((r.e~ ~y'e, Arts IPLZ-l..t: =: e\"Dl8::..r ::: ~~ l·D ~:\T~~,.'\.:J:::. :-"L';'IRJC:--:O :1...'\-n f..I,.C"""L n"! htrebr dtcl:m' ~hat the wcrt tr~m i l'd .. [a.(!d.t' .J..r ~ r. ,;. o::Mh:. ... P..e.~ .d ...... _......... _..... ................... !"i Ill)" " n~l!Ji\ l we.rk I han: c o, Or:'le- j n- ot::. ilDr othH S'tud .. n;:~ wort vr trow any other sourcE' __ t :': c~ p: \\h~lt dut refH t'o{'e or lC' t.no":lt'd~tI1l ..nt!i ~l dt t::pJ.i.C1tl~ · t.n [he te:;:l on ha~ anr part been ';\,III('n for Ill t by " o. -

Diseases Affecting Finfish
Diseases Affecting Finfish Legislation Ireland's Exotic / Disease Name Acronym Health Susceptible Species Vector Species Non-Exotic Listed National Status Disease Measures Bighead carp (Aristichthys nobilis), goldfish (Carassius auratus), crucian carp (C. carassius), Epizootic Declared Rainbow trout (Oncorhynchus mykiss), redfin common carp and koi carp (Cyprinus carpio), silver carp (Hypophtalmichthys molitrix), Haematopoietic EHN Exotic * Disease-Free perch (Percha fluviatilis) Chub (Leuciscus spp), Roach (Rutilus rutilus), Rudd (Scardinius erythrophthalmus), tench Necrosis (Tinca tinca) Beluga (Huso huso), Danube sturgeon (Acipenser gueldenstaedtii), Sterlet sturgeon (Acipenser ruthenus), Starry sturgeon (Acipenser stellatus), Sturgeon (Acipenser sturio), Siberian Sturgeon (Acipenser Baerii), Bighead carp (Aristichthys nobilis), goldfish (Carassius auratus), Crucian carp (C. carassius), common carp and koi carp (Cyprinus carpio), silver carp (Hypophtalmichthys molitrix), Chub (Leuciscus spp), Roach (Rutilus rutilus), Rudd (Scardinius erythrophthalmus), tench (Tinca tinca) Herring (Cupea spp.), whitefish (Coregonus sp.), North African catfish (Clarias gariepinus), Northern pike (Esox lucius) Catfish (Ictalurus pike (Esox Lucius), haddock (Gadus aeglefinus), spp.), Black bullhead (Ameiurus melas), Channel catfish (Ictalurus punctatus), Pangas Pacific cod (G. macrocephalus), Atlantic cod (G. catfish (Pangasius pangasius), Pike perch (Sander lucioperca), Wels catfish (Silurus glanis) morhua), Pacific salmon (Onchorhynchus spp.), Viral -

Colored Gemstones Cultured Pearls
Cultured Pearls Colored Gemstones Diamond Council of America ©2016 Cultured Pearls In This Lesson: •A World Apart • Pearl Traditions • Natural Pearls • Cultured Pearls •Value Factors •Product Highlights • Culturing Sales A WORLD APART In Lesson 1 you learned that any kind of gem except diamond is considered a colored gem. Although pearls are included in that broad classification, they really belong to a world apart. Most customers recognize this instinctively, sensing a special appeal about pearls. There are several themes you can use in a sales presenta- tion to evoke or enhance pearl’s separate place in the gem kingdom: • Pearls are born in water. This intuitive contrast with other gems, which are dug from the ground, gives pearls an aura of gentleness, freshness, and fluid grace. • Pearls originate from life. While most gems are minerals produced by inanimate geology, pearls are organic. They come from living beings. Much of pearls’ mystique arises from this connection. • Pearls possess a beauty that’s all their own. Most gems depend on cutting or carving to reveal their charms, but pearls emerge gleaming from their shells. Cultured pearls are born in water and originate from living organisms. They Though certain factors of pearl value are comparable are natural in their beauty and classic to those of other gems, key considerations are unique. as a gem. Colored Gemstones 5 1 Cultured Pearls Cultured pearls are modern forms of a classic gem. They ® combine Nature’s creative power with human art and JA SPC SKILLS If you’re participating in the JA® science. You could even say that cultured pearls show how Sales Professional Certification people can work with the environment to make age-old Program™, this lesson presents infor- mation related to the following Skill beauty available now, and for future generations as well. -

The Effect of Parasite Infection on Clam (Austrovenus Stutchburyi) Growth Rate and Body Condition in an Environment Modified by Commercial Harvesting
The effect of parasite infection on clam (Austrovenus stutchburyi) growth rate and body condition in an environment modified by commercial harvesting Sorrel O’Connell-Milne A thesis submitted for the degree of Masters of Science (Marine Science) University of Otago Dunedin, New Zealand May 2015 iii Abstract Commercial fishing often reduces densities and changes the structure of target populations. Within these target populations, the transmission dynamics of parasites are usually strongly dependent on host densities. Because parasites have direct impacts on their host and also indirect effects on interspecific interactions and ecosystem function, harvest-induced reductions in host density can have wide repercussions. The present research investigates the effect of commercial harvesting on echinostome parasite infection within the Otago clam fishery. Clams (Austrovenus stutchburyi) have been commercially harvested from Blueskin Bay since 1983, and since 2009, experimental harvesting has extended to beds within Otago Harbour. Parasite loads (numbers of echinostome metacercariae per clam) within areas of high fishing pressure were compared to adjacent unharvested areas to assess the effects of harvesting on parasite abundance in clams. At 14 sites within Blueskin Bay and Otago Harbour, a subset of shellfish of varying age and sizes were analysed for parasite load. Unparasitised juvenile clams were also collected and caged in situ over a winter and summer period to monitor spatial and temporal patterns of parasite infection. Finally, the effect of parasite load on the growth rate of clams was monitored both in situ and in a laboratory experiment, in which juvenile clams were infected with varying levels of parasite and mortality, body condition and foot length was quantified. -

An Assessment of Potential Heavy Metal Contaminants in Bivalve Shellfish from Aquaculture Zones Along the Coast of New South Wales, Australia
PEER-REVIEWED ARTICLE Hazel Farrell,* Phil Baker, Grant Webster, Food Protection Trends, Vol 38, No. 1, p. 18–26 Copyright© 2018, International Association for Food Protection Edward Jansson, Elizabeth Szabo and 6200 Aurora Ave., Suite 200W, Des Moines, IA 50322-2864 Anthony Zammit NSW Food Authority, 6 Ave. of the Americas, Newington NSW 2127, Australia An Assessment of Potential Heavy Metal Contaminants in Bivalve Shellfish from Aquaculture Zones along the Coast of New South Wales, Australia ABSTRACT INTRODUCTION Evaluation of shellfish aquaculture for potential contam- Certain elements are essential in human physiology; inants is essential for consumer confidence and safety. however, an incorrect balance or excess of certain elements Every three years, between 1999 and 2014, bivalve in the diet can result in negative health effects. Heavy metals shellfish from aquaculture zones in up to 31 estuaries are of particular concern because of their ability to persist across 2,000 km of Australia’s east coast were tested and accumulate in the environment. While heavy metals for cadmium, copper, lead, mercury, selenium and zinc. can occur naturally in the environment, human activities Inorganic arsenic was included in the analyses in 2002, and run-off from urban and agricultural land use may and total arsenic was used as a screen for the inorganic increase their concentrations (6, 29). This is particularly form in subsequent years. Concentrations of inorganic important when considering the growing demands on arsenic, cadmium, lead and mercury were low and did not coastal resources due to increasing populations (3) and the exceed maximum limits mandated in the Australia New ability of filter feeding bivalve shellfish to bio-accumulate Zealand Food Standards Code. -

National Review of Ostrea Angasi Aquaculture: Historical Culture, Current Methods and Future Priorities
National review of Ostrea angasi aquaculture: historical culture, current methods and future priorities Christine Crawford Institute of Marine and Antarctic Studies ! [email protected] " secure.utas.edu.au/profles/staff/imas/Christine-Crawford Executive summary Currently in Australia Ostrea angasi oysters (angasi) are being cultured on a small scale, with several farmers growing relatively small numbers of angasis on their predominately Sydney rock or Pacifc oyster farms. Very few farmers are culturing commercially viable quantities of angasi oysters. There are several reasons for this. Although angasi oysters occur in the intertidal zone, they are naturally most abundant in the subtidal and are less tolerant of fuctuating environmental conditions, especially temperature and salinity, than other oyster species. They also have a much shorter shelf life and start to gape after one to two days. Additionally, angasi oysters are susceptible to Bonamiosis, a parasitic disease which has caused major mortalities in several areas. Stress caused by extremes or a combination of factors such as high stocking densities, rough handling, poor food, high temperatures and low salinities have all been observed to increase the prevalence of Bonamiosis. Growth rates of angasi oysters have also been variable, ranging from two to four years to reach market size. From discussions with oyster famers, managers and researchers and from a review of the literature I suggest that the survival and growth of cultured angasi oysters could be signifcantly improved by altering some farm management practices. Firstly, growout techniques need to be specifcally developed for angasi oysters which maintain a low stress environment (not modifcations from other oysters). -

The Ecological and Socio-Cultural Values of Estuarine Shellfisheries in Hawai`I and Aotearoa New Zealand
‘Mauka makai’ ‘Ki uta ki tai’: The ecological and socio-cultural values of estuarine shellfisheries in Hawai`i and Aotearoa New Zealand. Ani Alana Kainamu Murchie Ngāpuhi, Ngāti Kahu ki Whangaroa, Cunningham Clan A thesis submitted in fulfilment of the requirements for the degree of Doctor of Philosophy in Environmental Science, The University of Canterbury 2017 Abstract Estuaries rank among the most anthropogenically impacted aquatic ecosystems on earth. There is a growing consensus on anthropogenic impacts to estuarine and coastal environments, and consequently the ecological, social, and cultural values. The protection of these values is legislated for within the U.S. and Aotearoa New Zealand (NZ). The respective environmental catchment philosophy ‘Mauka Makai’ and ‘Ki Uta Ki Tai’ (lit. inland to sea) of Indigenous Hawaiian and Ngāi Tahu forms the overarching principle of this study. The scientific component of this study measured shellfish population indices, condition index, tissue and sediment contamination which was compared across the landscape development index, physico-chemical gradient and management regimes. Within the socio-cultural component of this study, Indigenous and non-Indigenous local residents, ‘beach-goers’, managers, and scientists were interviewed towards their perception and experience of site and catchment environmental condition, resource abundance and changes, and management effectiveness of these systems. Both the ecological and cultural findings recognised the land as a source of anthropogenic stressors. In Kāne`ohe Bay, Hawai`i, the benthic infaunal shellfish density appears to be more impacted by anthropogenic conditions compared with the surface dwelling Pacific oyster, Crassostrea gigas. The latter was indicative of environmental condition. Although the shellfish fishery has remained closed since the 1970s, clam densities have continued to decline. -
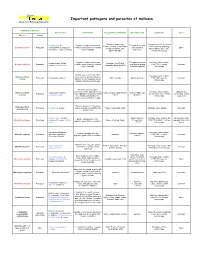
Pathogens Table 2006
Important pathogens and parasites of molluscs Genetic and Pathology Laboratory Pathogen or parasite Host species Host impact Geographical distribution Infection period Diagnostic Cycle Species Group Denmark, Netherland, Incubation period : 3-4 month Ostrea edulis, O. Parasite of oyster haemocytes Throughout the year France, Ireland, Great Britain in infected area. Histology, Bonamia ostreae Protozoan Conchaphila, O. Angasi, O. (=>all the tissues can be invaded). (with a peak in Direct (except Scotland), Italy, tissue imprint, PCR, ISH, Puelchana, Tiostrea chilensis Oyster mortality September) Spain and USA electron microscopy Parasite of oyster haemocytes Throughout the year Histology, tissue imprint, Ostrea angasi, Ostrea Australia, New Zéland, Bonamia exitiosa Protozoan (=>all the tissues can be invaded). (with a peak during PCR, ISH, electron Unknown chilensis, Ostrea edulis Tasmania, Spain (in 2007) Oyster mortality Australian autumn) microscopy Parasites present in connective Histology, tissue imprint, Haplosporidium tissue (mantle, gonads, digestive Protozoan Crassostrea virginica USA, Canada Spring-summer PCR, ISH, electron Unknown costale gland) but not in digestive tubule microscopy epithelia. Mortality in May-June Parasites present in gills, connective tissue. Sporulation only Histology, tissue imprint, Unknown but Haplosporidium Crassostrea virginica, USA, Canada, Japan, Korea, Summer (May until Protozoan in the epithelium of digestive gland smear, PCR, HIS, electron intermediate host is nelsoni Crassostrea gigas France October) in C. virginica, Mortalities in C. microscopy suspected virginica. No mortality in C. gigas Parasite present in connective Haplosporidium Protozoan O. edulis, O. angasi tissue. Sometimes, mortality can France, Netherland, Spain Histology, tissue imprint Unknown armoricanum be observed Ostrea edulis , Tiostrea Spring-summer Histology, tissue imprint, ISH, Intermediate host: Extracellular parasite of the Marteilia refringens Protozoan chilensis, O. -

FAO Fisheries Technical Paper 402/2
ISSNO0428-9345 FAO Asia Diagnostic Guide to FISHERIES TECHNICAL Aquatic Animal Diseases PAPER 402/2 NETWORK OF AQUACULTURE CENTRES IN ASIA-PACIFIC C A A N Food and Agriculture Organization of the United Nations A F O F S I I A N T P A ISSNO0428-9345 FAO Asia Diagnostic Guide to FISHERIES TECHNICAL Aquatic Animal Diseases PAPER 402/2 Edited by Melba G. Bondad-Reantaso NACA, Bangkok, Thailand (E-mail: [email protected]) Sharon E. McGladdery DFO-Canada, Moncton, New Brunswick (E-mail: [email protected]) Iain East AFFA, Canberra, Australia (E-mail: [email protected]) and Rohana P. Subasinghe NETWORK OF FAO, Rome AQUACULTURE CENTRES (E-mail: [email protected]) IN ASIA-PACIFIC C A A N Food and Agriculture Organization of the United Nations A F O F S I I A N T P A The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) or of the Network of Aquaculture Centres in Asia-Pa- cific (NACA) concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its fron- tiers or boundaries. ISBN 92-5-104620-4 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior permission of the copyright owner. -

Breeding and Domestication of Eastern Oyster (Crassostrea
W&M ScholarWorks VIMS Articles Virginia Institute of Marine Science 2014 Breeding And Domestication Of Eastern Oyster (Crassostrea Virginica) Lines For Culture In The Mid-Atlantic, Usa: Line Development And Mass Selection For Disease Resistance Anu Frank-Lawale Virginia Institute of Marine Science Standish K. Allen Jr. Virginia Institute of Marine Science Lionel Degremont Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/vimsarticles Part of the Marine Biology Commons Recommended Citation Frank-Lawale, Anu; Allen, Standish K. Jr.; and Degremont, Lionel, "Breeding And Domestication Of Eastern Oyster (Crassostrea Virginica) Lines For Culture In The Mid-Atlantic, Usa: Line Development And Mass Selection For Disease Resistance" (2014). VIMS Articles. 334. https://scholarworks.wm.edu/vimsarticles/334 This Article is brought to you for free and open access by the Virginia Institute of Marine Science at W&M ScholarWorks. It has been accepted for inclusion in VIMS Articles by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. Journal of Shellfish Research, Vol. 33, No. 1, 153–165, 2014. BREEDING AND DOMESTICATION OF EASTERN OYSTER (CRASSOSTREA VIRGINICA) LINES FOR CULTURE IN THE MID-ATLANTIC, USA: LINE DEVELOPMENT AND MASS SELECTION FOR DISEASE RESISTANCE ANU FRANK-LAWALE,* STANDISH K. ALLEN, JR. AND LIONEL DE´GREMONT† Virginia Institute of Marine Science, Aquaculture Genetics and Breeding Technology Center, College of William and Mary, 1375 Greate Road, Gloucester Point, VA 23062 ABSTRACT A selective breeding program for Crassostrea virginica was established in 1997 as part of an initiative in Virginia to address declining oyster harvests caused by the two oyster pathogens Haplosporidium nelsoni (MSX) and Perkinsus marinus (Dermo). -

Shellfish Reefs at Risk
SHELLFISH REEFS AT RISK A Global Analysis of Problems and Solutions Michael W. Beck, Robert D. Brumbaugh, Laura Airoldi, Alvar Carranza, Loren D. Coen, Christine Crawford, Omar Defeo, Graham J. Edgar, Boze Hancock, Matthew Kay, Hunter Lenihan, Mark W. Luckenbach, Caitlyn L. Toropova, Guofan Zhang CONTENTS Acknowledgments ........................................................................................................................ 1 Executive Summary .................................................................................................................... 2 Introduction .................................................................................................................................. 6 Methods .................................................................................................................................... 10 Results ........................................................................................................................................ 14 Condition of Oyster Reefs Globally Across Bays and Ecoregions ............ 14 Regional Summaries of the Condition of Shellfish Reefs ............................ 15 Overview of Threats and Causes of Decline ................................................................ 28 Recommendations for Conservation, Restoration and Management ................ 30 Conclusions ............................................................................................................................ 36 References ............................................................................................................................. -

Molecular Detection of Marteilia Sydneyi, Pathogen of Sydney Rock Oysters
DISEASES OF AQUATIC ORGANISMS Vol. 40: 137-146.2000 Published March 14 Dis Aquat Org ~ Molecular detection of Marteilia sydneyi, pathogen of Sydney rock oysters Sarah N. ~leeman'l*,Robert D. ~dlard~ '~epartmentof Parasitology, University of Queensland, Brisbane, Queensland 4072, Australia '~rotozoaSection, Queensland Museum, PO Box 3300, South Brisbane, Queensland 4101, Australia ABSTRACT: The life cycle of Marteilia sydneyi, the aetiological agent of QX disease in the Sydney rock oyster Saccostrea commercialis, is not known. We have developed and optirnised 2 diagnostic assays, the polymerase chain reaction (PCR) and in situ hybridisation, for use in investigating the role of pos- sible alternative hosts in the life cycle of this pathogen. PCR primers, designed within the ITS1 rDNA of M. sydneyi, amplified a 195 bp fragment. Sensitivity of the PCR assay was assessed using DNA extracted from known numbers of sporonts purified from infected oyster digestive gland. DNA equiva- lent to 0.01 sporonts was detectable following agarose gel electrophoresis. The potential inhibitory effect of the presence of host DNA on the PCR assay was tested by the addition of oyster genornic DNA during amplification. Concentrations of host DNA in excess of 50 ng per 20 p1 reaction reduced the sensitivity of the test. Environmental validation of the PCR assay was demonstrated by the amplifica- tion of M. sydneyl DNA from 50 ng of genomic DNA extracted from QX-infected oysters. A DNA probe was constructed using the M. sydneyi unique primers and was able to detect 10 pg of M. sydneyi PCR amplified DNA in dot-blot hybridisations. The probe hybridised with presporulating and sporulating M.