Potential Uses for Peroxymonosulfate in Pulping and Bleaching
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

4월 24일(목), 09:00~10:30
포스터 I: 4월 24일(목), 09:00~10:30 공업화학: 4월 24일(목), 09:00 ~ 10:30 좌장: 박정훈(동국대) Cleaning system study with mounting activated species of solution using P공업목-1(118) (강원대)권흥수, 이원규 atmospheric pressure plasma 기공구조 조절 제올라이트 촉매를 이용한 액체연료의 흡열량 향상 (고려대)현동훈, 김중연, 김유리, 전병희, P공업목-2(118) (우수 포스터 발표상 후보) 김성현, (국방과학(연))정병훈, 한정식 (고려대)신동건, 김성현, 이창훈, P공업목-3(118) 열 안정성이 우수한 CO 흡착제용 다공성 유무기 복합 지지체 제조 2 조동현, 정현철 (고려대)김유리, 김성현, 전병희, P공업목-4(119) 분산제의 구조에 따른 Jet A-1 연료의 열산화반응 탄소침적물 감소 연구 김중연, 현동훈 (국방과학(연))한정식, 정병훈 Effect of SAA Pretreatment on Temperature of Enzymatic Hydrolysis Using P공업목-5(119) (경기대)장서윤, 박장한, 김준석 Lignocellulosic Biomass Pretreatment and fractionation of Helianthus tuberosus residue by flow-through P공업목-6(119) (경기대)박용철, 박장한, 김준석 reaction (순천대)오성준 (순천대)양지선, 박권필 P공업목-7(119) Carbon Felt 산처리에 의한 바나듐 레독스 흐름전지에 대한 영향 ((주)CNL Energy)나일채, 이정훈 ((주)ETIS)추천호 메틸카바메이트 및 메탄올을 이용한 디메틸카보네이트 제조를 위한 고활성 P공업목-8(120) (한양대)이재홍, 서영웅 촉매 조사 (우수 포스터 발표상 후보) 연속흐름반응기에서 고농도 NaBH 가수분해 반응의 촉매 종류에 따른 P공업목-9(120) 4 (순천대)오성준, 정현승, 박권필 수소발생연구 (순천대)안명원, 곽동영, 복영빈, 박권필 P공업목-10(120) 해조류 종류별 후코산틴 추출 및 염분세척 ((주) ETIS)추천호, 김영숙 (순천대)이세훈, 박권필, ((주)ETIS)김영숙, P공업목-11(120) 효소연료전지에서 Anode 조건에 따른 OCV 변화 추천호, ((주)CNL Energy)나일채 (순천대)정재현, 정회범, 박권필 P공업목-12(121) PEMFC MEA제법에 의한 성능 및 내구성 향상 ((주)CNL Energy)라일채, 이 호 (순천대)정재현, 신은경, 정회범, 박권필 P공업목-13(121) PEM 수전해 과정에서 전극과 전해질 막의 변화 ((주)CNL Energy)라일채, 이 호 (순천대)정재현, 박권필 P공업목-14(121) 직접 개미산 연료전지(DFAFC)의 구동 조건에 따른 성능 비교 ((주)CNL Energy)나일채 (순천대)정재진, 박권필 P공업목-15(121) 가속화도 4.0의 전극, 막, MEA 가속화 기법 개발 (현대자동차)안병기, 김세훈, 고재준 (순천대)정재진, 박권필 P공업목-16(122) 탄화수소막을 사용한 -

Peroxy Compounds Human Health and Ecological Draft Risk Assessment DP 455445, 455446
Peroxy Compounds Human Health and Ecological Draft Risk Assessment DP 455445, 455446 UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, D.C. 20460 OFFICE OF CHEMICAL SAFETY AND POLLUTION PREVENTION MEMORANDUM Date: March 11, 2020 SUBJECT: Registration Review Draft Risk Assessment for the Peroxy Compounds PC Code: 000595, 063201, 063604, 063607, DP Barcode: 455445, 455446 063209, 128860 Decision No: 558073, 558074 Docket No: EPA-HQ-OPP-2009-0546 Regulatory Action: Registration Review Case No: 6059, 4072, 5081 Risk Assessment Type: DRA CAS No: 7722-84-1, 79-21-0, 33734-57-5, 15630-89-4, 10058-23-8, 70693-62-8 TO: Kendall Ziner, Chemical Review Manager Rick Fehir, Ph.D., Team Lead Rose Kyprianou, Branch Chief Regulatory Management Branch (RMB) II Antimicrobials Division (7510P) Office of Pesticide Programs FROM: Andrew Byro, Ph.D., Chemist Kathryn Korthauer, Biologist Timothy Dole, Industrial Hygienist Deborah Burgin, Ph.D., DABT, Toxicologist Risk Assessment and Science Support Branch Antimicrobials Division (7510P) Office of Pesticide Programs THROUGH: Judy Facey, Ph.D., Human Health Risk Assessment Process Leader MP for JF Diana Hsieh, Ecological Risk Assessment Process Leader MP for DH Timothy Leighton, Senior Science Advisor MP for TL Laura Parsons, Associate Branch Chief Melissa Panger, Ph.D., Branch Chief Risk Assessment and Science Support Branch Antimicrobials Division (7510P) This document provides the draft human health and ecological risk assessment conducted in support of the antimicrobial use sites of the following peroxy compounds: hydrogen peroxide, peracetic acid, peroxyoctanoic acid, and sodium percarbonate. Page 1 of 74 Peroxy Compounds Human Health and Ecological Draft Risk Assessment DP 455445, 455446 Although the peroxymonosulfate compounds were included in the peroxy compounds Final Work Plan (FWP), they will not be included in this risk assessment. -

Chemicals Used for Chemical Manufacturing Page 1 of 2
Chemicals used for Chemical Manufacturing Page 1 of 2 Acetic Acid (Glacial, 56%) Glycol Ether PMA Acetone Glycol Ether PNB Acrylic Acid Glycol Ether PNP Activated Carbon Glycol Ether TPM Adipic Acid Glycols Aloe Vera Grease Aluminum Stearate Gum Arabic Aluminum Sulfate Heat Transfer Fluids Amino Acid Heptane Ammonium Acetate Hexane Ammonium Bicarbonate Hydrazine Hydrate Ammonium Bifluoride Hydrochloric Acid (Muriatic) Ammonium Chloride Hydrogen Peroxide Ammonium Citrate Hydroquinone Ammonium Hydroxide Hydroxylamine Sulfate Ammonium Laureth Sulfate Ice Melter Ammonium Lauryl Sulfate Imidazole Ammonium Nitrate Isobutyl Acetate Ammonium Persulfate Isobutyl Alcohol Ammonium Silicofluoride Calcium Stearate Dipropylene Glycol Isopropanolamine Ammonium Sulfate Carboxymethylcellulose Disodium Phosphate Isopropyl Acetate Antifoams Caustic Potash D'Limonene Isopropyl Alcohol Antifreeze Caustic Soda (All Grades) Dodecylbenzene Sulfonic Acid Isopropyl Myristate Antimicrobials Caustic Soda (Beads, Prills) (DDBSA) Isopropyl Palmitate Antimony Oxide Cetyl Alcohol Dowfrost Itaconic Acid Aqua Ammonia Cetyl Palmitate Dowfrost HD Jojoba Oil Ascorbic Acid Chlorine, Granular Dowtherm SR-1 Keratin Barium Carbonate Chloroform Dowtherm 4000 Lactic Acid Barium Chloride Chromic Acid EDTA Lanolin Beeswax Citric Acid (Dry and Liquid) EDTA Plus Lauric Acid Bentonite Coal Epsom Salt Lauryl Alcohol Benzaldehyde Cocamide DEA Ethyl Acetate Lecithin Benzoic Acid Copper Nitrate Ethyl Alcohol (Denatured) Lime Benzyl Alcohol Copper Sulfate Ethylene Glycol Linoleic Acid Bicarbonate -

Ammonium Persulfate
SAFETY DATA SHEET Ammonium Persulfate Section 1. Identification GHS product identifier : Ammonium Persulfate Code : 76322 Other means of : ammonium persulphate; Peroxydisulfuric acid, diammonium salt; diammonium identification peroxodisulfate; Diammonium persulfate Supplier/Manufacturer : 3420 Central Expressway, Santa Clara CA 95051 In case of emergency : Chemtrec: 1 800 424 9300 Outside USA & Canada: +1 703 527 3887 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : OXIDIZING SOLIDS - Category 3 substance or mixture ACUTE TOXICITY (oral) - Category 4 SKIN CORROSION/IRRITATION - Category 2 SERIOUS EYE DAMAGE/ EYE IRRITATION - Category 2 RESPIRATORY SENSITIZATION - Category 1 SKIN SENSITIZATION - Category 1 SPECIFIC TARGET ORGAN TOXICITY (SINGLE EXPOSURE) (Respiratory tract irritation) - Category 3 GHS label elements Hazard pictograms : Signal word : Danger Hazard statements : May intensify fire; oxidizer. Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause allergy or asthma symptoms or breathing difficulties if inhaled. May cause an allergic skin reaction. May cause respiratory irritation. Precautionary statements Prevention : Wear protective gloves. Wear eye or face protection. In case of inadequate ventilation wear respiratory protection. Keep away from heat. - No smoking. Keep away from clothing, incompatible materials and combustible materials. Take any precaution to avoid mixing with combustibles and other incompatible materials. Use only outdoors or in a well-ventilated area. Avoid breathing dust. Do not eat, drink or smoke when using this product. Wash hands thoroughly after handling. Contaminated work clothing should not be allowed out of the workplace. Response : IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing. -

Preparing to Manufacture Hydrogen Peroxide
PREPARING TO MANUFACTURE HYDROGEN PEROXIDE Part of the Hydrogen Peroxide Propulsion Guide The early laboratory preparation of hydrogen peroxide was based on the technique that Thenard used during the initial preparation of hydrogen peroxide. In this technique, barium nitrate, purified by recrystallization, was decomposed by heating in air in a porcelain retort. The resulting oxide was further oxidized by heating in a stream of oxygen to a dull red heat. The barium peroxide which formed was then dampened, ground, and dissolved in hydrochloric acid (nitric acid was used in Thenard’s initial experiments). A slight excess of sulfuric acid was then added to precipitate barium sulfate and regenerate hydrochloric acid. The procedure of barium peroxide solution and sulfate precipitation was repeated several times in the same solution to increase the peroxide concentration (concentrations of up to 33 percent by weight hydrogen peroxide could be achieved in this manner). The concentrated solution containing water, hydrogen peroxide, and hydrochloric acid, along with accumulated impurities, was cooled with ice and saturated with barium peroxide; iron and manganese impurities in the solution were then precipitated out as phosphates. The hydrochloric acid was removed by the addition of silver sulfate and the sulfate ion was removed by the subsequent addition of barium oxide. Further concentration was accomplished by vacuum distillation until “no further density increase occurs.” Thenard reported that 100 w/o hydrogen peroxide (on the basis of density data and the measurement of the volume of oxygen released) could be obtained by this technique. The first record of commercial production of hydrogen peroxide appeared in the 1865 to 1875 period. -

Acrylamide Polymerization — a Practical Approach
electrophoresis tech note 1156 Acrylamide Polymerization — A Practical Approach Paul Menter, Bio-Rad Laboratories, 2000 Alfred Nobel Drive, Polyacrylamide Gel Polymerization Hercules, CA 94547 USA AcrylamideBis Polyacrylamide Introduction The unparalleled resolution and flexibility possible with CH2 CH + CH2 CH CH2 CH CH2 CH CH2 CH polyacrylamide gel electrophoresis (PAGE) has led to its CO CO CO CO CO widespread use for the separation of proteins and nucleic NH2 NH NH2 NH2 NH acids. Gel porosity can be varied over a wide range to meet CH2 CH2 specific separation requirements. Electrophoresis gels and NH NH NH NH buffers can be chosen to provide separation on the basis of CO 2 2 CO CO C O charge, size, or a combination of charge and size. CH2 CH CH2 CH CH2 CH CH2 CH The key to mastering this powerful technique lies in the polymerization process itself. By understanding the important Purity of Gel-Forming Reagents parameters, and following a few simple guidelines, the novice Acrylamide can become proficient and the experienced user can optimize Gel-forming reagents include the monomers, acrylamide and bis, separations even further. as well as the initiators, usually ammonium persulfate and TEMED or, occasionally, riboflavin and TEMED. On a molar This bulletin takes a practical approach to the preparation of basis, acrylamide is by far the most abundant component in the polyacrylamide gels. Its purpose is to provide the information monomer solution. As a result, acrylamide may be the primary required to achieve reproducible, controllable polymerization. source of interfering contaminants (Dirksen and Chrambach For those users interested only in the “bare essentials,” the 1972). -

(Ph.D.) Environmental Engineering
UNIVERSITY OF CINCINNATI Date: June 15, 2005 I, Georgios Anipsitakis , hereby submit this work as part of the requirements for the degree of: Doctorate of Philosophy (Ph.D.) in: Environmental Engineering It is entitled: Cobalt/Peroxymonosulfate and Related Oxidizing Reagents for Water Treatment This work and its defense approved by: Chair: Dr. Dionysios Dionysiou Dr. Paul Bishop Dr. George Sorial Dr. Souhail Al-Abed COBALT/PEROXYMONOSULFATE AND RELATED OXIDIZING REAGENTS FOR WATER TREATMENT A dissertation submitted to the Division of Research and Advanced Studies of the University of Cincinnati in partial fulfillment of the requirements for the degree of DOCTORATE OF PHILOSOPHY (Ph.D.) in the Department of Civil and Environmental Engineering of the College of Engineering 2005 by Georgios P. Anipsitakis Diploma (B.S./M.S.), Chemical Engineering, Nat. Technical Univ. Athens, 2000 Committee Chair: Dr. Dionysios D. Dionysiou ii Abstract This dissertation explores the fundamentals of a novel advanced oxidation technology, the II cobalt/peroxymonosulfate (Co /KHSO5) reagent, for the treatment of persistent and hazardous II substances in water. Co /KHSO5 is based on the chemistry of the Fenton Reagent and proceeds via the generation of sulfate radicals, which similarly to hydroxyl radicals, readily attack and degrade organic and microbial contamination in water. Very few studies have exploited the reactivity of sulfate radicals for environmental applications. Compared to the extensively investigated hydroxyl radicals, sulfate radicals are not fully understood. Following this approach, the coupling of nine transition metals with hydrogen peroxide (H2O2), potassium peroxymonosulfate (KHSO5) and persulfate (K2S2O8) was also explored. The objective was again the generation of inorganic radicals and the efficient degradation of organic contaminants in water. -

Microchip Design for Chemiluminesence Detection
Double spiral detection channel for on-chip chemiluminescence detection Author Lok, Khoi Seng, Kwok, Yien Chian, Nam-Trung, Nguyen Published 2012 Journal Title Sensors and Actuators B: Chemical DOI https://doi.org/10.1016/j.snb.2012.04.047 Copyright Statement © 2012 Elsevier B.V.. This is the author-manuscript version of this paper. Reproduced in accordance with the copyright policy of the publisher. Please refer to the journal's website for access to the definitive, published version. Downloaded from http://hdl.handle.net/10072/50085 Griffith Research Online https://research-repository.griffith.edu.au Lab on a Chip for Chemiluminescence Detection Khoi Seng Lok1, Yien Chian Kwok1* and Nam Trung Nguyen2 1National Institute of Education, Nanyang Technological University, Nanyang Walk, Singapore 637616, Singapore. E-mail: [email protected] 2School of Mechanical and Production Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore 639798, Singapore. E-mail: [email protected] Abstracts In this paper reports a three layered device that consists of a double spiral channel design for chemiluminsence (CL) detection and a passive micromixer to facilitate mixing of reagents. Compared to the design with a single spiral channel, the design with two overlapping spiral channels doubled the CL intensity emitted from the reaction. Furthermore, the addition of the passive micromixer improved the signal by 1.5 times. Luminol-based chemiluminescence reaction was used for the characterization of the device. This device was successfully used for the determination of L-cysteine and uric acid. Keywords: chemiluminescence, lab-on-chip, micro total analysis system, luminol, cobalt, L-cysteine, uric acid 1 Introduction Chemiluminescence (CL) detection methods do not require an excitation light source. -

Most Common Substances in Chemicals for Different Industries
Table 14 Most common substances by industry Figures for 2009. Substances contained in more than 10 products. A substance can be omitted for secrecy reasons. Industry CAS-no Substance Number of products A01 Agriculture 7732-18-5 Water 458 6484-52-2 Nitric acid ammonium salt 77 57-55-6 1,2-Propanediol 75 64742-65-0 Distillates (petroleum), solvent-dewaxed heavy 74 paraffinic 63148-62-9 Siloxanes and Silicones, di-Me 60 57-13-6 Urea 54 67-63-0 2-Propanol 54 11138-66-2 Xanthan gum 53 7783-20-2 Sulfuric acid diammonium salt 53 7722-76-1 Phosphoric acid, monoammonium salt 52 7487-88-9 Sulfuric acid magnesium salt (1:1) 51 7447-40-7 Potassium chloride 46 56-81-5 1,2,3-Propanetriol 44 7778-80-5 Sulfuric acid dipotassium salt 43 1310-73-2 Sodium hydroxide 41 7647-14-5 Sodium chloride 41 2634-33-5 1,2-Benzisothiazol-3(2H)-one 38 1310-58-3 Potassium hydroxide 33 7757-79-1 Nitric acid potassium salt 33 7785-87-7 Manganese sulfate 32 7664-38-2 Phosphoric acid 32 72623-87-1 Lubricating oils, petroleum, C20-50, 31 hydrotreated neutral oil-based 64741-89-5 Distillates (petroleum), solvent-refined light 29 paraffinic 77-92-9 1,2,3-Propanetricarboxylic acid, 2-hydroxy- 28 8061-51-6 Lignosulfonic acid, sodium salt 28 7778-18-9 Sulfuric acid, calcium salt (1:1) 27 64-17-5 Ethanol 27 14807-96-6 Talc 26 64742-94-5 Solvent naphtha (petroleum), heavy arom. 25 68649-42-3 Phosphorodithioic acid, O,O-di-C1-14-alkyl 24 esters, zinc salts (2:1) 64-02-8 Glycine, N,N'-1,2-ethanediylbis[N- 24 (carboxymethyl)-, tetrasodium salt 64-19-7 Acetic acid 23 99-76-3 Benzoic acid, -

Ac. H' UY1 : T 'I 7 April23,2004
F'll'J 'J.'t/032.770 i!,P,P, it> 0~ -'II-~ I ;J..¾,5 Aece; 11(:c/. WDNR., O'f/?.1 I01i Ac. h' UY1 : t 'I 7 April23,2004 Ms. Pam Mylotta Wisconsin Department of Natural Resources 2300 North Martin Luther King Jr. Street P.O. Box 12436 Milwaukee, Wisconsin 53212-0436 Re: Request for Source Area Chemical Injection Permit C & D Technologies, Inc. 900 Keefe A venue Milwaukee, Wisconsin 53212-1709 Dear Ms. Mylotta: On behalf of the Johnson Controls Battery Group, Inc. (JCBGI) and C & D Technologies, Inc. (C&D), MWH Americas, Inc. (MWH) has prepared this request for an injection permit for groundwater treatment in the acid unloading area at the above referenced site. The purpose of the permit request is to provide information to the Wisconsin Department of Natural Resources (WDNR) for approval to conduct a pilot study and full-scale injection of sodium persulfate into the acid unloading area for treatment of volatile organic compounds (VOCs) in groundwater. BACKGROUND INFORMATION Soil and groundwater contamination were found to exist on the Facility property exceeding State environmental standards, based on the August 1999 Phase II Environmental Site Assessment (ESA) of the Facility. In May and June 2001, remedial action was completed for the on-site lead, PCB, and VOC impacted soils. A summary of the remedial activities is contained in the February 2002 On Site Remedial Actions Construction Completion Report. Approximately 400 tons of VOC impacted soil was removed during this investigation. Temporary wells were installed in July 2001 and November 2001 as part of the groundwater VOC characterization investigations documented in the June 2001 and October 2001 Work Plans, respectively. -
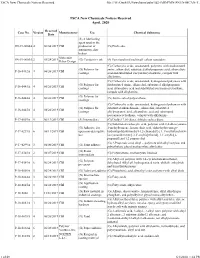
TSCA New Chemicals Notices Received File:///W:/Oneepa/Newchems/Pubs/5D2-IMD/PMN-SNUN-MCAN-T
TSCA New Chemicals Notices Received file:///W:/OneEPA/Newchems/pubs/5d2-IMD/PMN-SNUN-MCAN-T... TSCA New Chemicals Notices Received April, 2020 Received Case No. Version Manufacturer Use Chemical Substance Date (S) A lubricating agent used in the SN-19-0004A 4 06/04/2019 CBI production of (G) Pitch coke automotive disc brakes Molecular SN-19-0005A 2 05/28/2019 (G) Conductive ink (S) Functionalized multiwall carbon nanotubes Rebar Design (G) Carboxylic acids, unsaturated, polymers with disubstituted (G) Polymer for amine, alkanediol, substituted alkylpropanoic acid, alkanedioic P-16-0442A 4 06/26/2019 CBI coatings acid and substituted isocyanatocycloalkane, compds with alkylamine (G) Carboxylic acids, unsaturated, hydrogenated polymers with (G) Polymer for disubstituted amine, alkanediol, substituted alkylpropanoic P-16-0443A 4 06/26/2019 CBI coatings acid, alkanedioic acid and substituted isocyanatocycloalkane, compds with alkylamine (G) Polymer for P-16-0444A 4 06/26/2019 CBI (G) Amine salted polyurethane coatings (G) Carboxylic acids, unsaturated, hydrogenated polymers with (G) Polymer for substituted alkanediamine, alkanediol, substituted P-16-0445A 4 06/26/2019 CBI coatings alkylpropanoic acid, alkanedioic acid and substituted isocyanatocycloalkane, compds with alkylamine P-17-0007A 5 06/13/2019 CBI (S) Intermediate (G) Dialkyl 7,10-dioxa, dithiahexadeca diene (G) Substituted carboxylic acid, polymer with 2,4-diisocyanato- (G) Adhesive for 1-methylbenzene, hexanedioic acid, alpha-hydro-omega- P-17-0239A 6 06/11/2019 CBI open non-descriptive -

Chemical Analyses for Selected Minor Elements in Pierre Shale
Chemical Analyses for Selected Minor Elements in Pierre Shale GEOLOGICAL SURVEY PROFESSIONAL PAPER 391-A Chemical Analyses for Selected Minor Elements in Pierre Shale By L. F. RADER and F. S. GRIMALDI ANALYTICAL METHODS IN GEOCHEMICAL INVESTIGATIONS OF THE PIERRE SHALE GEOLOGICAL SURVEY PROFESSIONAL PAPER 391-A UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1961 UNITED STATES DEPARTMENT OF THE INTERIOR STEW ART L. UDALL, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director For sale by the Superintendent of Documents, U.S. Government Printing Office Washington 25, D.C. CONTENTS Page Page Abstract--_________________________________________ A-l Methods for the determination of individual Introduction ___-----_-_--_________________-__--__-_ 1 constituents Continued General remarks._________________________ ______ 1 Copper lead, and zinc Continued Laboratories. -._---____-_-______________________ 2 Zinc, dithizone method._____^_______________ A-20 Acknowledgments. ___-_-_-_--______--_-_________ 2 Lead, dithizone method______------_-____-__- 22 Division of work________________________________ 2 Arsenic.___________,__________-_-_--___-__---_- 23 Methods selected and treatment of data ___________ 3 Acid digestion, heteropoly blue method________ 23 Sample preparation _________________________________ 4 Alternative fusion-heteropoly blue method_____ 24 Methods for the determination of individual constituents. 4 Titanium. _____________________________________ 4 Selenium, distillation, visual-estimation method.____ 25 Peroxide method._--_--_-____._____________ 4 Molybdenum and tungsten______-____------_---_- 27 Alternative tiron method, ___________________ 5 Isolation by method 1, alpha-benzoinoxime pre Vanadium. _--.---_____-_-___.__________________ 7 cipitation. ___----_-_-_-__--_----_-----_-- 27 Fusion-leach separation method. _____________ 7 Isolation by alternative method 2, alpha-ben Alternative cupferron separation method......