While There Are Many Thousands of Different Chemical Compounds There
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemical Chemical Hazard and Compatibility Information
Chemical Chemical Hazard and Compatibility Information Acetic Acid HAZARDS & STORAGE: Corrosive and combustible liquid. Serious health hazard. Reacts with oxidizing and alkali materials. Keep above freezing point (62 degrees F) to avoid rupture of carboys and glass containers.. INCOMPATIBILITIES: 2-amino-ethanol, Acetaldehyde, Acetic anhydride, Acids, Alcohol, Amines, 2-Amino-ethanol, Ammonia, Ammonium nitrate, 5-Azidotetrazole, Bases, Bromine pentafluoride, Caustics (strong), Chlorosulfonic acid, Chromic Acid, Chromium trioxide, Chlorine trifluoride, Ethylene imine, Ethylene glycol, Ethylene diamine, Hydrogen cyanide, Hydrogen peroxide, Hydrogen sulfide, Hydroxyl compounds, Ketones, Nitric Acid, Oleum, Oxidizers (strong), P(OCN)3, Perchloric acid, Permanganates, Peroxides, Phenols, Phosphorus isocyanate, Phosphorus trichloride, Potassium hydroxide, Potassium permanganate, Potassium-tert-butoxide, Sodium hydroxide, Sodium peroxide, Sulfuric acid, n-Xylene. Acetone HAZARDS & STORAGE: Store in a cool, dry, well ventilated place. INCOMPATIBILITIES: Acids, Bromine trifluoride, Bromine, Bromoform, Carbon, Chloroform, Chromium oxide, Chromium trioxide, Chromyl chloride, Dioxygen difluoride, Fluorine oxide, Hydrogen peroxide, 2-Methyl-1,2-butadiene, NaOBr, Nitric acid, Nitrosyl chloride, Nitrosyl perchlorate, Nitryl perchlorate, NOCl, Oxidizing materials, Permonosulfuric acid, Peroxomonosulfuric acid, Potassium-tert-butoxide, Sulfur dichloride, Sulfuric acid, thio-Diglycol, Thiotrithiazyl perchlorate, Trichloromelamine, 2,4,6-Trichloro-1,3,5-triazine -

United States Patent Office Patented Dec
3,481,871 United States Patent Office Patented Dec. 2, 1969 1. 3,481,871 DTHOETHANE, DERVATIVES AND ORGANIC COMPOSTIONS CONTAINING THE SAME Herbert Myers, Barrington, and Alfred P. Kozacik, Woodbury, N.J., assignors to Mobil Oil Corpo ration, a corporation of New York No Drawing. Filed Apr. 24, 1967, Ser. No. 632,907 Int. CI. C10m 1/38, 3/32; C07c. 149/12 U.S. C. 252-45 2 Claims O and ABSTRACT OF THE DISCLOSURE Novel organo-sulfur derivatives are produced by re acting a mercaptain with a sulfur chloride compound, reacting the resulting organic sulfenyl or thiosulfenyl 5 chloride with an olefin, and finally reacting the resulting SSR, product with a metal sulfur-containing salt. The final product may be used in lubricating oils and other in wherein R, R2, and x have the above definitions, and X dustrial fluids as a load-carrying additive. is oxygen or sulfur, Y is oxygen, sulfur or nitrogen and 20 R is hydrogen, or alkyl, cycloalkyl, aralkyl, aryl or alkaryl having from 1 to 20 carbon atoms. Rb may also FIELD OF INVENTION contain nitrogen, oxygen- or sulfur-containing Substi This invention relates to organic compositions con tletS. taining novel organo-sulfur derivatives of dithioethanes, and to a method of producing these derivatives. 25 DESCRIPTION OF SPECIFIC EMBODIMENTS PRIOR ART The products of this invention are produced by react In U.S. Patents Nos. 2,708,199 and 2,886,593, specific ing (1) a mercaptan, RSH, with (2) a sulfur halide. The sulfur compounds have been disclosed. In the first-named resulting organosulfenyl halide is then reacted with (3) patent, there is disclosed a method of producing organic 3) an olefin, CRR2=CHP, to produce an organo thioethyl polysulfides by reacting an olefin having from 6 to 30 halide intermediate. -

Ionic & Covalent Compound Naming Race
Name __________________________ Date ___________ Period _________ Ionic & Covalent Compound Naming Race First, identify whether these compounds are ionic or covalent. Then, use the correct formula writing rules to write the correct chemical formulas for each compound. Type of Compound: Compound Name Chemical Formula Ionic or Covalent 1) copper (II) chlorite 2) sodium hydroxide 3) nitrogen dioxide 4) cobalt (III) oxalate 5) ammonium sulfide 6) aluminum cyanide 7) carbon disulfide 8) tetraphosphorous pentoxide 9) potassium permanganate 10) manganese (III) chloride Type of Compound: Compound Name Chemical Formula Ionic or Covalent 11) calcium bromate 12) carbon monoxide 13) potassium oxide 14) antimony tribromide 15) zinc phosphate 16) copper (II) bicarbonate 17) dinitrogen tetroxide 18) manganese (IV) carbonate 19) lead (IV) nitride 20) pentacarbon decahydride Ionic_Covalent Names: Chapter 9 Honors Chemistry Name __________________________ Date ___________ Period _________ Ionic & Covalent Compound Naming First, identify whether these compounds are ionic or covalent. Then, use the correct naming rules to write the correct names for each compound. Chemical Formula Type of Compound: Compound Name Ionic or Covalent 21) CdBr2 22) Cr(Cr2O7)3 23) SBr2 24) (NH4)2CrO4 25) CuO 26) Pt3(PO3)4 27) Al(ClO4)3 28) NH3 29) Ca(C2H3O2)2 30) N2O Type of Compound: Chemical Formula Compound Name Ionic or Covalent 31) V(SO4)2 32) Ag2CO3 33) N2S3 34) FeSO3 35) Zn(NO2)2 36) C6H12O6 37) PCl3 38) Mn(OH)7 39) Ni(NO3)2 40) O2 Ionic_Covalent Names: Chapter 9 Honors Chemistry Name __________________________ Date ___________ Period _________ Compound Naming Race - Solutions Be the first team in the room to correctly get all the names on this sheet right. -

Formula of a Compound
Name__________________________________ Formula of a Compound 1. Useful only if it correctly represents the substance. 2. The composition is determined in chemical analysis. 3. The formula then is derived by atomic theory and chemical bonding methods. 4. A chemical formula is a qualitative and quantitative description of the composition of a pure substance, either as element or a compound. Naming Binary Compounds Two types of Binary Compounds A. Combining a metal and a non-metal 1. The metallic element is usually named and written first-this is at times called the cation. Example: KCl -The cation is K, so the name Potassium is written 2. The non-metal, which is called the anion, is made from the root word of the name of the element plus an -ide ending. Cl is chlorine, the root is chlor, then add the -ide ending to write the word chloride. 3. Place both names together: Potassium chloride Problem Section #1 Directions: Write the non-metal ion names for the following 1. Nitrogen __________________ 2. Oxygen ___________________ 3. Sulfur ____________________ 4. Fluorine __________________ 5. Chlorine __________________ 6. Bromine __________________ Problem Section #2 Directions: Write the correct names for the following Binary Compounds. Formula Ions and charge Name KCl K+ Cl- Potassium chloride CaCl2 AlCl3 Na2S BaS Al2S3 CsF Ba3N2 MgBr2 Rb2O Writing Chemical Formulas 1. Write the symbols to the element with the appropriate charge. 2. Cross over the number that was written as a charge. Example Calcium chloride 2+ - Ca Cl becomes CaCl2 Problem Section #3 Directions: Write the correct formula for the following Binary Compounds. -
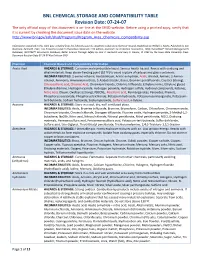
BNL CHEMICAL STORAGE and COMPATIBILITY TABLE Revision Date: 07-24-07 the Only Official Copy of This Document Is On-Line at the SHSD Website
BNL CHEMICAL STORAGE AND COMPATIBILITY TABLE Revision Date: 07-24-07 The only official copy of this document is on-line at the SHSD website. Before using a printed copy, verify that it is current by checking the document issue date on the website. http://www.bnl.gov/esh/shsd/Programs/Program_Area_Chemicals_Compatibility.asp Information contained in this table was compiled from the following sources: Academic Laboratory Chemical Hazards Guidebook by William J. Mahn, Published by Van Nostrand, Reinhold, 1991; Fire Protection Guide to Hazardous Materials 11th edition, National Fire Protection Association, 1994; Hazardtext® Hazard Managements Database; INFOTEXT® Documents Database; Better Science Through Safety by Jack A. Gerlovich and Gary E. Downs, © 1981 by the Iowa State University Press. Document Revision Date 07-24-07 Ken Erickson CHO Chemical Chemical Hazard and Compatibility Information Acetic Acid HAZARDS & STORAGE: Corrosive and combustible liquid. Serious health hazard. Reacts with oxidizing and alkali materials. Keep above freezing point (62 ºF) to avoid rupture of carboys and glass containers. INCOMPATIBILITIES: 2-amino-ethanol, Acetaldehyde, Acetic anhydride, Acids, Alcohol, Amines, 2-Amino- ethanol, Ammonia, Ammonium nitrate, 5-Azidotetrazole, Bases, Bromine pentafluoride, Caustics (strong), Chlorosulfonic acid, Chromic Acid, Chromium trioxide, Chlorine trifluoride, Ethylene imine, Ethylene glycol, Ethylene diamine, Hydrogen cyanide, Hydrogen peroxide, Hydrogen sulfide, Hydroxyl compounds, Ketones, Nitric Acid, Oleum, Oxidizers -
Standard X-Ray Diffraction Powder Patterns
E^l Admin. NBS MONOGRAPH 25—SECTION 5 Refecii^M not to be ^ferlrom the library. Standard X-ray Diffraction Powder Patterns ^\ / U.S. DEPARTMENT OF COMMERCE S NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS The National Bureau of Standards^ provides measurement and technical information services essential to the efficiency and effectiveness of the work of the Nation's scientists and engineers. The Bureau serves also as a focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To accomplish this mission, the Bureau is organized into three institutes covering broad program areas of research and services: THE INSTITUTE FOR BASIC STANDARDS . provides the central basis within the United States for a complete and consistent system of physical measurements, coordinates that system with the measurement systems of other nations, and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation's scientific community, industry, and commerce. This Institute comprises a series of divisions, each serving a classical subject matter area: —Applied Mathematics—Electricity—Metrology—Mechanics—Heat—Atomic Physics—Physical Chemistry—Radiation Physics— -Laboratory Astrophysics^—Radio Standards Laboratory,^ which includes Radio Standards Physics and Radio Standards Engineering—Office of Standard Refer- ence Data. THE INSTITUTE FOR MATERIALS RESEARCH . conducts materials research and provides associated materials services including mainly reference materials and data on the properties of ma- terials. Beyond its direct interest to the Nation's scientists and engineers, this Institute yields services which are essential to the advancement of technology in industry and commerce. -

Report-Of Committee on Chemicals and Explosives
448 REPORT OF COMMITTEE ON CHEMICALS AND EXPLOSIVES CE-1 Report-of Committee on Chemicals and Explosives Correlating Committee Dr. Robert W. Van Dolah, Chairman, Pittsburgh Mining and Safety Research Center, Bureau of Mines, U.S. Department of the Interior, 4800 Forbes Ave., Pittsburgh, PA 15213 Chester I. Babeock,~ Secretary, National Pire Protection Assn., 470 Atlantic Ave., Boston, MA 02210 W. H. Doyle, Simsbury, CT ilenry T. Rlttman, Institute of Makers of •, Thomas E. Duke, Fire Prevention & Engi- Explosives neering Bureau of Texas Richard F. Schwab, Allied Chemical Corp. Dr. Richard Y. Le Vine, Olin Corp. tNonvoting. Sectional Committee on Electrical Equipment in Chemical Atmospheres Dr. Richard Y. Le Vine, Chairman, Olin Corp., 120 Long Ridge Rd., Stamford, CT 06904 Chester I. Babcock,~ Secretary, National Fire Protection Association, 470 Atlantic Ave., Boston, MA 02210 L. J. Hall. Panel No. 14, National Electrical R. F. Schwab, Morristown, NJ Code Committee W. A. Short, National Electrical-Manu- • Robert P. llowell, American Petroleu~i In" facturers Assn. stitute George O. Hunt, Jr., Manufacturing Chem- Alternates. ists' Assn. Elton L. Lltehfleld, Pittsburgh, PA F. D. Alroth. (Alternate to P. J. Schram) Frederick L. Maltby, Instrument Society W. Calder (Alternate to F. L. Maltby) of America W. H. Levers (Alternate to Robert P. C. E. Miller, Norwood, MA Howell) " Frank E. Rademacher, Chicago, IL J. Rennle (Alternate to C. E. Miller) John E. Rogerson. Cincinnati, OH Thomas S. Staron, (Alternate to Frank E. P. J. Schram, Chicago, IL Rademaehcr) tNonvoting 449 CE-2 EXPLANATION OF REPORT Sectional Committee on llazardous Chemical Reactions R. F. Schwab, Chairman, Allied Chemical Corp., P.O. -

Table of Contents
TABLE OF CONTENTS 1. Introduction 1 A. Organization 1 B. Goals 1 C. Key Responsibilities 1 D. Facilities 2 2. Objectives 2 3. General Safety Information 3 A. Lab Protocol 3 B. Recommended Lab Techniques 4 4. Emergency Procedures and Equipment 9 Primary Emergency Procedures 10 Special Procedures for Radioactive Hazards 11 C. Special Procedures for Biological Hazards 11 D. Building Evacuation Procedures 12 E. Emergency Equipment 13 5. Hazard Communication Act 15 A. Requirements 15 B. Material Safety Data Sheets 15 6. Chemical Hazards and Control 19 A. Chemical Categories and Use and Storage 19 Flammables 20 Oxidizers 23 Corrosives 25 Reactives 27 Compressed Gas Cylinders 32 B. Personal Protective Clothing 32 C. Chemical Safety Equipment 35 7. Biological Hazards and Control 39 A. Recommended Laboratory Practices 40 B. Laboratory Equipment 37 C. Personal Protective Clothing 43 D. Waste Disposal 43 E. Bloodborne Pathogens 43 F. Laboratory Animals 44 G. Biosafety Levels 45 Biosafety Level 1 45 Biosafety Level 2 47 Biosafety Level 3 49 H. Emergency Procedures 52 8. Radiation Hazards and Control 54 9. Controlled Substances, Precursor Chemicals and Chemical Laboratory Apparatus 55 A. List of Precursor Items 55 B. Responsibility 56 C. Purchase Orders 56 D. Surplus Property 56 E. Security Procedures Governing Use of Controlled Items 56 F. Site Security 56 G. Operational Security 57 H. Inventory and Reporting of Loss 57 I. Designation of a University Liaison 57 10. Chemical Waste 58 A. Hazardous Wastes 58 B. Regulated Wastes 58 C. Gas Cylinders 58 D. Containers 59 E. Broken Glassware 59 F. PCB Light Ballasts 59 G. -

Lawrence Berkeley National Laboratory Recent Work
Lawrence Berkeley National Laboratory Recent Work Title REFERENCES TO ULTRAVIOLET PHOTOELECTRON SPECTRA OF GASEOUS MOLECULES Permalink https://escholarship.org/uc/item/3bf9869z Authors Horne, D.H. Jolly, W.L. Publication Date 1986-09-01 eScholarship.org Powered by the California Digital Library University of California DISCLAIMER This document was prepared as an account of work sponsored by the United States Government. While this document is believed to contain COlTect information, neither the United States Government nor any agency thereof, nor the Regents of the University of California, nor any of their employees, makes any walTanty, express or implied, or assumes any legal responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, proCess, or service by its trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof, or the Regents of the University of California. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof or the Regents of the University of California. , References to Ultraviolet Photoelectron Spectra of Gaseous Molecules By Dorothy H. Horne and William L. Jolly Materials and Molecular Research Division, Lawrence Berkeley Laboratory, University of California, Berkeley, California 94720 This report consists of an alphabetical listing of compounds together with Chemical Abstracts references (for the years 1972-1985) to articles describing research in which these compounds are the subject of gas-phase ultraviolet photoelectron spectroscopic studies. -

Thermodynamic Properties of Minerals and Related Substances at 298.15°K (25.0°C) and One Atmosphere (1.013 Bars) Pressure and at Higher Temperatures
Thermodynamic Properties of Minerals and Related ~ Substances at 298.15°K (25.0°C) o and One Atmosphere (1.013 Bars) hS O Pressure and at Higher Temperatures bd 82 GEOLOGICAL SURVEY BULLETIN 1259 ORTON MEMORIAL LIBRARY i: '£ OHIO STATE UWVEHSITY JUN 1 6 2000 CO a I to Or Thermodynamic Properties of Minerals and Related Substances at 298.15°K (25.0°C) and One Atmosphere (1.013 Bars) Pressure and at Higher Temperatures By RICHARD A. ROBIE ajid DAVID R. WALDBAUM GEOLOGICAL SURVEY BULLETIN 1259 A summary of the thermodynamic data for minerals at 298.15°K together with calculated values of the functions AH°f,T, AGI,T, ST, and (Gr H^.s/T) at temperatures up to 2,000°K ORTON MEMORIAL LIBRARY THE OHIO STATE UNIVERSITY 155 S. OVAL DRIVE UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1968 UNITED STATES DEPARTMENT OF THE INTERIOR WALTER J. HICKEL, Secretary GEOLOGICAL SURVEY William T. Pecora, Director Library of Congress catalog-card No. OS 68-223 First printing 1968 Second printing 1970 For sale by the Superintendent of Documents, U.S. Government Printing OflBlce Washington, D.C. 20402 - Price $1.25 (paper cover) CONTENTS Page Abstract _____________________________________ 1 Introduction ___________________________________... 1 Acknowledgments ________________________________ 2 Physical constants and atomic weights ________________ 3 Reference states and transitions _________,______________ 5 Sources of data ___________,________________,______ 5 Methods of calculation ______________________________ 8 Thermodynamic properties at 298.15°K ___________________ 11 Thermodynamic properties at high temperatures ______________ 26 References and notes _______________________________ 238 Index of names ___________________________________ 249 Index of formulas ________________________________ 253 TABLES Page TABLE 1. -

Aspen Plus7 10 STEADY STATE SIMULATION
Aspen Plus7 10 STEADY STATE SIMULATION Version Physical Property Data AspenTech7 REFERENCE MANUAL COPYRIGHT 1981—1999 Aspen Technology, Inc. ALL RIGHTS RESERVED The flowsheet graphics and plot components of Aspen Plus were developed by MY-Tech, Inc. Aspen Aerotran, Aspen Pinch, ADVENT®, Aspen B-JAC, Aspen Custom Modeler, Aspen Dynamics, Aspen Hetran, Aspen Plus®, AspenTech®, B-JAC®, BioProcess Simulator (BPS), DynaPlus, ModelManager, Plantelligence, the Plantelligence logo, Polymers Plus®, Properties Plus®, SPEEDUP®, and the aspen leaf logo are either registered trademarks, or trademarks of Aspen Technology, Inc., in the United States and/or other countries. BATCHFRAC and RATEFRAC are trademarks of Koch Engineering Company, Inc. Activator is a trademark of Software Security, Inc. Rainbow SentinelSuperPro is a trademark of Rainbow Technologies, Inc. Élan License Manager is a trademark of Élan Computer Group, Inc., Mountain View, California, USA. Microsoft Windows, Windows NT, Windows 95 and Windows 98 are either registered trademarks or trademarks of Microsoft Corporation in the United States and/or other countries. All other brand and product names are trademarks or registered trademarks of their respective companies. The License Manager portion of this product is based on: Élan License Manager © 1989-1997 Élan Computer Group, Inc. All rights reserved Use of Aspen Plus and This Manual This manual is intended as a guide to using Aspen Plus process modeling software. This documentation contains AspenTech proprietary and confidential information and may not be disclosed, used, or copied without the prior consent of AspenTech or as set forth in the applicable license agreement. Users are solely responsible for the proper use of Aspen Plus and the application of the results obtained.