Rangia Cuneata) Ecological Risk Screening Summary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

No. 8. September 2014
No 8, September 2014 News from the NOBANIS secretariat The secretary at NOBANIS Christina Fevejle Nielsen, started on her new job in the Nature Agency, Danish Ministry of Environment in December 2013, and Maya Helene Quaade Caspersen has since January 2014 been the new secretary at NOBANIS, with a current contract until February 2015. Maya has a master degree in biology from the University of Copenhagen, and has experience with IAS from working with regulation in Denmark. As the new secretary of NOBANIS, I would like to take this opportunity to thank all partners for welcoming me, and for the good collaboration the last couple of months. This year I will continue the work with revising the database that Helene started and finish the update of the remaining fact sheets. Another project that will have my attention in 2014 is the ATAN project “Achieving the Aichi Target 9, IAS in the Nordic and Baltic region”. Read more about the project further down. 10 years with NOBANIS (2004-2014) This year NOBANIS can celebrate its 10th anniversary. Here is a short summary with background information and achievements of the network (2004-2014) The Nordic-Baltic Network on Invasive Alien Species (NOBANIS) was initiated as a project funded by the Nordic Council of Ministers in 2004 to fulfilled parts of the CBD Decision VI/23, Guiding Principles for the prevention, introduction and mitigation of impacts of alien species that threaten ecosystems, habitats or species. The aim of the project was to develop a regional, distributed and interoperable network on the critical issue of invasive alien species (IAS) in the marine, freshwater and terrestrial environments and their consequences for biological diversity, the cultural landscape and outdoor life. -

Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora)
Gulf of Mexico Science Volume 34 Article 4 Number 1 Number 1/2 (Combined Issue) 2018 Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Laguna Madre, Tamaulipas, Mexico: Spatial and Temporal Distribution Martha Reguero Universidad Nacional Autónoma de México Andrea Raz-Guzmán Universidad Nacional Autónoma de México DOI: 10.18785/goms.3401.04 Follow this and additional works at: https://aquila.usm.edu/goms Recommended Citation Reguero, M. and A. Raz-Guzmán. 2018. Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Laguna Madre, Tamaulipas, Mexico: Spatial and Temporal Distribution. Gulf of Mexico Science 34 (1). Retrieved from https://aquila.usm.edu/goms/vol34/iss1/4 This Article is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Gulf of Mexico Science by an authorized editor of The Aquila Digital Community. For more information, please contact [email protected]. Reguero and Raz-Guzmán: Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Lagu Gulf of Mexico Science, 2018(1), pp. 32–55 Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Laguna Madre, Tamaulipas, Mexico: Spatial and Temporal Distribution MARTHA REGUERO AND ANDREA RAZ-GUZMA´ N Molluscs were collected in Laguna Madre from seagrass beds, macroalgae, and bare substrates with a Renfro beam net and an otter trawl. The species list includes 96 species and 48 families. Six species are dominant (Bittiolum varium, Costoanachis semiplicata, Brachidontes exustus, Crassostrea virginica, Chione cancellata, and Mulinia lateralis) and 25 are commercially important (e.g., Strombus alatus, Busycoarctum coarctatum, Triplofusus giganteus, Anadara transversa, Noetia ponderosa, Brachidontes exustus, Crassostrea virginica, Argopecten irradians, Argopecten gibbus, Chione cancellata, Mercenaria campechiensis, and Rangia flexuosa). -

Gametogenic Cycle of <I>Rangia Cuneata</I> (Mactridae, Mollusca
BULLETIN OF MARINE SCIENCE, 45(1): 130-138, 1989 GAMETOGENIC CYCLE OF RANGIA CUNEATA (MACTRIDAE, MOLLUSCA) IN MOBILE BAY, ALABAMA, WITH COMMENTS ON GEOGRAPHIC VARIATION M. C. Jovanovich and K. R. Marion ABSTRACT Stages of the gametogenic cycle of the brackish water clam Rangia cuneata were investigated in Mobile Bay, Alabama, by histological examination of the gonads. Water temperature and salinity measurements were related to the gametogenic cycle and compared to those made in other studies on the same species in different locations. Clams in the early active phase were found from February through April, and those in the late active phase predominated in May. Gonads were ripe from July through September and were partially spawned from September through October. Clams became spent between November and January. Changes in meat, gonad, and total wet weight reflected the stages of the gametogenic cycle, while length of the shells remained nearly constant year round. The gametogenic cycle of R. cuneata in Mobile Bay followed a similar pattern to that observed in clams studied in other locations, except that gametogenesis began earlier, during late winter, and clams became spent earlier in the fall. It is suggested that the interactive effects of temperature, salinity, and nutrients can account for the differences in the timing and length of the stages of the gametogenic cycle between locations. Rangia cuneata (Gray) is a brackish water clam (Family Mactridae) abundant in the estuaries of the Gulf of Mexico. It is also found throughout the coastal areas of the eastern United States as far north as the upper Chesapeake Bay (Woodburn, 1962; Pfitzenmeyer, 1970). -

Environmental DNA Detection of the Invasive Mussel Mytella Strigata As a Surveillance Tool
Management of Biological Invasions (2021) Volume 12, Issue 3: 578–598 CORRECTED PROOF Research Article Environmental DNA detection of the invasive mussel Mytella strigata as a surveillance tool Zhi Ting Yip1,*, Chin Sing Lim2, Ywee Chieh Tay3, Yong How Jonathan Tan4, Stephen Beng5, Karenne Tun4, Serena Lay-Ming Teo2 and Danwei Huang1,2,6 1Department of Biological Sciences, National University of Singapore, Singapore 117558, Singapore 2Tropical Marine Science Institute, National University of Singapore, Singapore 119227, Singapore 3Temasek Life Sciences Laboratory, Singapore 117604, Singapore 4National Biodiversity Centre, National Parks Board, Singapore 259569, Singapore 5Marine Conservation Group, Nature Society (Singapore), Singapore 389466, Singapore 6Centre for Nature-based Climate Solutions, National University of Singapore, Singapore 117558, Singapore Author e-mails: [email protected] (ZTY), [email protected] (CSL), [email protected] (YCT), [email protected] (YHJT), [email protected] (SB), [email protected] (KT), [email protected] (SLMT), [email protected] (DH) *Corresponding author Citation: Yip ZT, Lim CS, Tay YC, Tan YHJ, Beng S, Tun K, Teo SLM, Huang D Abstract (2021) Environmental DNA detection of the invasive mussel Mytella strigata as a The American charru mussel Mytella strigata (Hanley, 1843) is an invasive species surveillance tool. Management of of great concern along the shores of North America and Asia. As with most invasive Biological Invasions 12(3): 578–598, mussels, it is very difficult to eradicate once established. Surveillance therefore plays https://doi.org/10.3391/mbi.2021.12.3.05 a vital role in controlling its spread. Molecular tools like environmental DNA Received: 27 July 2020 (eDNA) have proved to be useful in recent years to assist in the early detection and Accepted: 7 February 2021 management of invasive species, with considerable advantages over conventional Published: 19 April 2021 methods like substrate monitoring and sampling, which can be relatively laborious and time-intensive. -

Rangia Cuneata (Mollusca: Bivalvia), in Northwestern France
Aquatic Invasions (2020) Volume 15, Issue 3: 367–381 CORRECTED PROOF Research Article Establishment and population features of the non-native Atlantic rangia, Rangia cuneata (Mollusca: Bivalvia), in northwestern France Robin Faillettaz1,2, Christophe Roger2,3, Michel Mathieu2,3, Jean Paul Robin2,3 and Katherine Costil2,3,* 1University of Miami Rosenstiel School of Marine & Atmospheric Science, 4600 Rickenbacker Causeway, Miami, FL 33149-1098, USA 2BOREA (Biologie des Organismes et Ecosystèmes Aquatiques); MNHN, UPMC, UCN, CNRS-7208, IRD-207; Université de Caen Normandie, Esplanade de la Paix, 14032 Caen Cedex 5, France 3Normandie Université, F-14032 Caen, France Author e-mails: [email protected] (RF), [email protected] (CR), [email protected] (MM), [email protected] (JPR), [email protected] (KC) *Corresponding author Citation: Faillettaz R, Roger C, Mathieu M, Robin JP, Costil K (2020) Establishment Abstract and population features of the non-native Atlantic rangia, Rangia cuneata (Mollusca: The presence of shells of the Atlantic rangia, Rangia cuneata, a brackish-water Bivalvia), in northwestern France. Aquatic species native from the Gulf of Mexico also known as gulf wedge clam, was Invasions 15(3): 367–381, https://doi.org/10. reported in 2017 on the French coasts of the English Channel, in the waterway that 3391/ai.2020.15.3.02 connects Caen to the sea. However, no information was available on whether a Received: 21 November 2019 population of this alien species had successfully established in the region. Here, Accepted: 20 March 2020 only empty shells—except for one live individual—were sampled in that waterway, Published: 29 April 2020 and the sampling was shifted to the nearby marina of Ouistreham, where water is mesohaline (6.89 ± SD 0.06 PSU). -

Limited Success of the Non-Indigenous Bivalve Clam
Oceanologia (2019) 61, 341—349 Available online at www.sciencedirect.com ScienceDirect j ournal homepage: www.journals.elsevier.com/oceanologia/ ORIGINAL RESEARCH ARTICLE Limited success of the non-indigenous bivalve clam Rangia cuneata in the Lithuanian coastal waters of the Baltic Sea and the Curonian Lagoon a,b, a a Sabina Solovjova *, Aurelija Samuilovienė , Greta Srėbalienė , a,c a Dan Minchin , Sergej Olenin a Marine Research Institute, Klaipėda University, Klaipėda, Lithuania b Department of Environmental Research, Environmental Protection Agency, Klaipėda, Lithuania c Marine Organism Investigations, Ballina, Killaloe, Ireland Received 29 January 2018; accepted 29 January 2019 Available online 13 February 2019 KEYWORDS Summary The gulf wedge clam, common rangia Rangia cuneata, with a native origin in the Gulf of Mexico has spread to north European brackish and freshwaters. This semitropical species is able to Semitropical bivalve; survive in conditions of low winter temperatures in boreal environment of the Baltic Sea. Its expansion Coastal lagoon; within lagoons and sheltered bays in the southern and eastern parts of the Baltic Sea appears to be Exposed coast; with natural spread and its discontinuous distribution is likely to have been with shipping, either Winter conditions; within ballast water or as settled stages transported with dredged material. In this account, we report Ballast water; on the occurrence of R. cuneata in Lithuanian waters. We compare habitats of the common rangia in Natural spread. the Curonian Lagoon and in the exposed coastal waters of the Baltic Sea. We notice high mortality of the species in the Lithuanian waters in comparison to the neighboring Vistula Lagoon. Based on finding of small specimens of R. -

The Evolution of Extreme Longevity in Modern and Fossil Bivalves
Syracuse University SURFACE Dissertations - ALL SURFACE August 2016 The evolution of extreme longevity in modern and fossil bivalves David Kelton Moss Syracuse University Follow this and additional works at: https://surface.syr.edu/etd Part of the Physical Sciences and Mathematics Commons Recommended Citation Moss, David Kelton, "The evolution of extreme longevity in modern and fossil bivalves" (2016). Dissertations - ALL. 662. https://surface.syr.edu/etd/662 This Dissertation is brought to you for free and open access by the SURFACE at SURFACE. It has been accepted for inclusion in Dissertations - ALL by an authorized administrator of SURFACE. For more information, please contact [email protected]. Abstract: The factors involved in promoting long life are extremely intriguing from a human perspective. In part by confronting our own mortality, we have a desire to understand why some organisms live for centuries and others only a matter of days or weeks. What are the factors involved in promoting long life? Not only are questions of lifespan significant from a human perspective, but they are also important from a paleontological one. Most studies of evolution in the fossil record examine changes in the size and the shape of organisms through time. Size and shape are in part a function of life history parameters like lifespan and growth rate, but so far little work has been done on either in the fossil record. The shells of bivavled mollusks may provide an avenue to do just that. Bivalves, much like trees, record their size at each year of life in their shells. In other words, bivalve shells record not only lifespan, but also growth rate. -
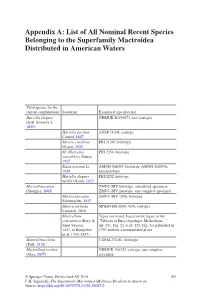
List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters
Appendix A: List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters Valid species (in the current combination) Synonym Examined type material Harvella elegans NHMUK 20190673, two syntypes (G.B. Sowerby I, 1825) Harvella pacifica ANSP 51308, syntype Conrad, 1867 Mactra estrellana PRI 21265, holotype Olsson, 1922 M. (Harvella) PRI 2354, holotype sanctiblasii Maury, 1925 Raeta maxima Li, AMNH 268093, lectotype; AMNH 268093a, 1930 paralectotype Harvella elegans PRI 2252, holotype tucilla Olsson, 1932 Mactrellona alata ZMUC-BIV, holotype, articulated specimen; (Spengler, 1802) ZMUC-BIV, paratype, one complete specimen Mactra laevigata ZMUC-BIV 1036, holotype Schumacher, 1817 Mactra carinata MNHN-IM-2000-7038, syntypes Lamarck, 1818 Mactrellona Types not found, based on the figure of the concentrica (Bory de “Tableau of Encyclopedique Methodique…” Saint Vincent, (pl. 251, Fig. 2a, b, pl. 252, Fig. 2c) published in 1827, in Bruguière 1797 without a nomenclatorial act et al. 1791–1827) Mactrellona clisia USNM 271481, holotype (Dall, 1915) Mactrellona exoleta NHMUK 196327, syntype, one complete (Gray, 1837) specimen © Springer Nature Switzerland AG 2019 103 J. H. Signorelli, The Superfamily Mactroidea (Mollusca:Bivalvia) in American Waters, https://doi.org/10.1007/978-3-030-29097-9 104 Appendix A: List of All Nominal Recent Species Belonging to the Superfamily… Valid species (in the current combination) Synonym Examined type material Lutraria ventricosa MCZ 169451, holotype; MCZ 169452, paratype; -

The Ocean Genome: Conservation and the Fair, Equitable and Sustainable Use of Marine Genetic Resources
Commissioned by BLUE PAPER The Ocean Genome: Conservation and the Fair, Equitable and Sustainable Use of Marine Genetic Resources LEAD AUTHORS Robert Blasiak, Rachel Wynberg, Kirsten Grorud-Colvert and Siva Thambisetty CONTRIBUTING AUTHORS Narcisa M. Bandarra, Adelino V.M. Canário, Jessica da Silva, Carlos M. Duarte, Marcel Jaspars, Alex D. Rogers, Kerry Sink and Colette C.C. Wabnitz oceanpanel.org About the High Level Panel for a Sustainable Ocean Economy The High Level Panel for a Sustainable Ocean Economy (Ocean Panel) is a unique initiative by 14 world leaders who are building momentum for a sustainable ocean economy in which effective protection, sustainable production and equitable prosperity go hand in hand. By enhancing humanity’s relationship with the ocean, bridging ocean health and wealth, working with diverse stakeholders and harnessing the latest knowledge, the Ocean Panel aims to facilitate a better, more resilient future for people and the planet. Established in September 2018, the Ocean Panel has been working with government, business, financial institutions, the science community and civil society to catalyse and scale bold, pragmatic solutions across policy, governance, technology and finance to ultimately develop an action agenda for transitioning to a sustainable ocean economy. Co-chaired by Norway and Palau, the Ocean Panel is the only ocean policy body made up of serving world leaders with the authority needed to trigger, amplify and accelerate action worldwide for ocean priorities. The Ocean Panel comprises members from Australia, Canada, Chile, Fiji, Ghana, Indonesia, Jamaica, Japan, Kenya, Mexico, Namibia, Norway, Palau and Portugal and is supported by the UN Secretary-General's Special Envoy for the Ocean. -

Southeastern Regional Taxonomic Center South Carolina Department of Natural Resources
Southeastern Regional Taxonomic Center South Carolina Department of Natural Resources http://www.dnr.sc.gov/marine/sertc/ Southeastern Regional Taxonomic Center Invertebrate Literature Library (updated 9 May 2012, 4056 entries) (1958-1959). Proceedings of the salt marsh conference held at the Marine Institute of the University of Georgia, Apollo Island, Georgia March 25-28, 1958. Salt Marsh Conference, The Marine Institute, University of Georgia, Sapelo Island, Georgia, Marine Institute of the University of Georgia. (1975). Phylum Arthropoda: Crustacea, Amphipoda: Caprellidea. Light's Manual: Intertidal Invertebrates of the Central California Coast. R. I. Smith and J. T. Carlton, University of California Press. (1975). Phylum Arthropoda: Crustacea, Amphipoda: Gammaridea. Light's Manual: Intertidal Invertebrates of the Central California Coast. R. I. Smith and J. T. Carlton, University of California Press. (1981). Stomatopods. FAO species identification sheets for fishery purposes. Eastern Central Atlantic; fishing areas 34,47 (in part).Canada Funds-in Trust. Ottawa, Department of Fisheries and Oceans Canada, by arrangement with the Food and Agriculture Organization of the United Nations, vols. 1-7. W. Fischer, G. Bianchi and W. B. Scott. (1984). Taxonomic guide to the polychaetes of the northern Gulf of Mexico. Volume II. Final report to the Minerals Management Service. J. M. Uebelacker and P. G. Johnson. Mobile, AL, Barry A. Vittor & Associates, Inc. (1984). Taxonomic guide to the polychaetes of the northern Gulf of Mexico. Volume III. Final report to the Minerals Management Service. J. M. Uebelacker and P. G. Johnson. Mobile, AL, Barry A. Vittor & Associates, Inc. (1984). Taxonomic guide to the polychaetes of the northern Gulf of Mexico. -

Common Rangia
- REFERENCE COPY Do Not Remove from the Librorv - U. S. Fish and Wildlife hirn ~iologicalReport 82 (11- 31 ) lvorlonolWetlands Research Cenwr TR EL-$2-4 April, 1986 700 Cajun Dome Boulevarrf Latayette,I - Louisiana 70506 Species Profiles: Life Histories and Environmental Requirements of Coastal Fishes and Invertebrates (Gulf of Mexico) COMMON RANGIA Coastal Ecology Group a Fish and Wildlife Service Watenvavs Ex~erimentStation U.S. Department of the Interior U.S. Army Corps of Engineers This is one of the first reports to be published in the new "Biological Report" series. This technical report series, published by the Research and Development branch of the U.S. Fish and Wildlife Service, replaces the "FWS/OBS1' series published from 1976 to September 1984. The Biolog- ical Report series is designed for the rapid publication of reports with an application orientation, and it continues the focus of the FWS/OBS series on resource management issues and fish and wi Id1 i fe needs. Biological Report 82(11.31) TR EL-82-4 April 1985 Species Profiles: Life Histories and Environmental Requirements of Coastal Fisheries and Invertebrates (Gulf of Mexico) COMMON RANG IA Mark W. LaSalle and Armando A. de la Cruz Department of Biological Sciences P.O. Drawer GY Mississippi State University Mississippi State, MS 39762 Project Officer John Parsons National Coastal Ecosystems Team U.S. Fish and Wildlife Service 1010 Gause Boulevard Sl idell, LA 70458 Performed for Coastal Ecology Group Waterways Experiment Station U.S. Army Corps of Engineers Vicksburg, MS 39180 and National Coastal Ecosystems Team Division of Biological Services Research and Development Fish and Wildlife Service U.S. -

Noaa 13648 DS1.Pdf
r LOAI<CO Qpy N Guide to Gammon Tidal IVlarsh Invertebrates of the Northeastern Gulf of IVlexico by Richard W. Heard UniversityofSouth Alabama, Mobile, AL 36688 and CiulfCoast Research Laboratory, Ocean Springs, MS39564" Illustrations by rimed:tul""'"' ' "=tel' ""'Oo' OR" Iindu B. I utz URt,i',"::.:l'.'.;,',-'-.,":,':::.';..-'",r;»:.",'> i;."<l'IPUS Is,i<'<i":-' "l;~:», li I lb~'ab2 Thisv,ork isa resultofreseaich sponsored inpart by the U.S. Department ofCommerce, NOAA, Office ofSea Grant, underGrani Nos. 04 8 Mol 92,NA79AA D 00049,and NA81AA D 00050, bythe Mississippi Alabama SeaGrant Consortium, byche University ofSouth Alabama, bythe Gulf Coast Research Laboratory, andby the Marine EnvironmentalSciences Consortium. TheU.S. Government isauthorized toproduce anddistribute reprints forgovern- inentalpurposes notwithstanding anycopyright notation that may appear hereon. *Preseitt address. This Handbook is dedicated to WILL HOLMES friend and gentleman Copyright! 1982by Mississippi hlabama SeaGrant Consortium and R. W. Heard All rightsreserved. No part of thisbook may be reproduced in any manner without permissionfrom the author. Printed by Reinbold Lithographing& PrintingCo., BooneviBe,MS 38829. CONTENTS 27 PREFACE FamilyMysidae OrderTanaidacea Tanaids!,....... 28 INTRODUCTION FamilyParatanaidae........, .. 29 30 SALTMARSH INVERTEBRATES ., FamilyApseudidae,......,... Order Cumacea 30 PhylumCnidaria =Coelenterata!......, . FamilyNannasticidae......,... 31 32 Class Anthozoa OrderIsopoda Isopods! 32 Fainily Edwardsiidae. FamilyAnthuridae