Therapy of Leprosy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Folic Acid Antagonists: Antimicrobial and Immunomodulating Mechanisms and Applications
International Journal of Molecular Sciences Review Folic Acid Antagonists: Antimicrobial and Immunomodulating Mechanisms and Applications Daniel Fernández-Villa 1, Maria Rosa Aguilar 1,2 and Luis Rojo 1,2,* 1 Instituto de Ciencia y Tecnología de Polímeros, Consejo Superior de Investigaciones Científicas, CSIC, 28006 Madrid, Spain; [email protected] (D.F.-V.); [email protected] (M.R.A.) 2 Consorcio Centro de Investigación Biomédica en Red de Bioingeniería, Biomateriales y Nanomedicina, 28029 Madrid, Spain * Correspondence: [email protected]; Tel.: +34-915-622-900 Received: 18 September 2019; Accepted: 7 October 2019; Published: 9 October 2019 Abstract: Bacterial, protozoan and other microbial infections share an accelerated metabolic rate. In order to ensure a proper functioning of cell replication and proteins and nucleic acids synthesis processes, folate metabolism rate is also increased in these cases. For this reason, folic acid antagonists have been used since their discovery to treat different kinds of microbial infections, taking advantage of this metabolic difference when compared with human cells. However, resistances to these compounds have emerged since then and only combined therapies are currently used in clinic. In addition, some of these compounds have been found to have an immunomodulatory behavior that allows clinicians using them as anti-inflammatory or immunosuppressive drugs. Therefore, the aim of this review is to provide an updated state-of-the-art on the use of antifolates as antibacterial and immunomodulating agents in the clinical setting, as well as to present their action mechanisms and currently investigated biomedical applications. Keywords: folic acid antagonists; antifolates; antibiotics; antibacterials; immunomodulation; sulfonamides; antimalarial 1. -

Chapter 3 Bacterial and Viral Infections
GBB03 10/4/06 12:20 PM Page 19 Chapter 3 Bacterial and viral infections A mighty creature is the germ gain entry into the skin via minor abrasions, or fis- Though smaller than the pachyderm sures between the toes associated with tinea pedis, His customary dwelling place and leg ulcers provide a portal of entry in many Is deep within the human race cases. A frequent predisposing factor is oedema of His childish pride he often pleases the legs, and cellulitis is a common condition in By giving people strange diseases elderly people, who often suffer from leg oedema Do you, my poppet, feel infirm? of cardiac, venous or lymphatic origin. You probably contain a germ The affected area becomes red, hot and swollen (Ogden Nash, The Germ) (Fig. 3.1), and blister formation and areas of skin necrosis may occur. The patient is pyrexial and feels unwell. Rigors may occur and, in elderly Bacterial infections people, a toxic confusional state. In presumed streptococcal cellulitis, penicillin is Streptococcal infection the treatment of choice, initially given as ben- zylpenicillin intravenously. If the leg is affected, Cellulitis bed rest is an important aspect of treatment. Where Cellulitis is a bacterial infection of subcutaneous there is extensive tissue necrosis, surgical debride- tissues that, in immunologically normal individu- ment may be necessary. als, is usually caused by Streptococcus pyogenes. A particularly severe, deep form of cellulitis, in- ‘Erysipelas’ is a term applied to superficial volving fascia and muscles, is known as ‘necrotiz- streptococcal cellulitis that has a well-demarcated ing fasciitis’. This disorder achieved notoriety a few edge. -

Leprosy – Eliminated and Forgotten: a Case Report Shiva Raj K.C.1,5* , Geetika K.C.1, Purnima Gyawali2, Manisha Singh3 and Milesh Jung Sijapati4
K.C. et al. Journal of Medical Case Reports (2019) 13:276 https://doi.org/10.1186/s13256-019-2198-1 CASE REPORT Open Access Leprosy – eliminated and forgotten: a case report Shiva Raj K.C.1,5* , Geetika K.C.1, Purnima Gyawali2, Manisha Singh3 and Milesh Jung Sijapati4 Abstract Background: Leprosy is a disease that was declared eliminated in 2010 from Nepal; however, new cases are diagnosed every year. The difficulty arises when the presentation of the patient is unusual. Case presentation: In this case report we present a case of a 22-year-old Tamang man, from the Terai region of Nepal, with a clinical presentation of fever, malaise, and arthralgia for the past 2 weeks with hepatosplenomegaly and bilateral cervical, axillary, and inguinal lymphadenopathy. Features of chronic inflammation with elevated erythrocyte sedimentation rate of 90 mm/hour and liver enzymes were noted. With no specific investigative findings, a diagnosis of Still’s disease was made and he was given prednisolone. On tapering the medication, after 2 weeks, the lymphadenopathy and fever reappeared. On biopsy of a lymph node, diagnosis of possible tuberculosis was made. On that basis anti-tuberculosis treatment category I was started. During his hospital stay, our patient developed nodular skin rashes on his shoulder, back, and face. The biopsy of a skin lesion showed erythema nodosum leprosum and he was diagnosed as having lepromatous leprosy with erythema nodosum leprosum; he was treated with anti-leprosy medication. Conclusion: An unusual presentations of leprosy may delay its prompt diagnosis and treatment; thus, increasing morbidity and mortality. -

Lepromatous Leprosy Simulating Sweet Syndrome
ISSN: 2469-5750 Zemmez et al. J Dermatol Res Ther 2018, 4:056 DOI: 10.23937/2469-5750/1510056 Volume 4 | Issue 1 Journal of Open Access Dermatology Research and Therapy CASE REPORT Lepromatous Leprosy Simulating Sweet Syndrome Youssef Zemmez1*, Ahmed Bouhamidi1, Salwa Belhabib2, Rachid Frikh1, Mohamed Boui1 1 and Naoufal Hjira Check for updates 1Department of Dermatology-Venereology, Mohammed V Military Training Hospital, Rabat, Morocco 2Department of Pathological Anatomy, Mohammed V Military Training Hospital, Rabat, Morocco *Corresponding author: Youssef Zemmez, Department of Dermatology-Venereology, Mohammed V Military Training Hospital, Rabat, Morocco, Tel: 0658150805, E-mail: [email protected] Abstract Leprosy or Hansen's disease is an infection by Mycobac- terium leprae (M. leprae), whose prevalence has consid- erably decreased since the application of the new anti-lep- rosy strategies advocated since 1982 by the World Health Organization (WHO). However, in the endemic countries several cases of leprosy are reported annually. We report a clinical case of lepromatous leprosy revealed by dissemi- nated maculopapular lesions simulating a Sweet syndrome highlighting the importance of knowing how to evoke this diagnosis in patients from endemic areas. Keywords Lepromatous leprosy, Rash, Sweet syndrome Introduction Lepromatous leprosy is generally manifested by Figure 1: a) Maculopapular erythematous lesions of the non-inflammatory lesions, hypochromic macules, and face; b) Papulonodular erythematous lesions in forearms progressive erythematous papulo-nodules. We report and hands. an observation of lepromatous leprosy in a 62-year-old patient from rural Morocco who was diagnosed with abdomen (Figure 2a and Figure 2b). The neurological maculopapular lesions. examination showed hypoesthesia in gloves and socks, with bilateral hypertrophy of the ulnar nerve. -
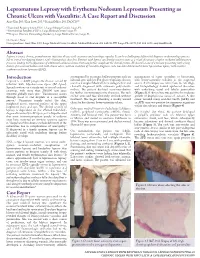
Lepromatous Leprosy with Erythema Nodosum Leprosum Presenting As
Lepromatous Leprosy with Erythema Nodosum Leprosum Presenting as Chronic Ulcers with Vasculitis: A Case Report and Discussion Anny Xiao, DO,* Erin Lowe, DO,** Richard Miller, DO, FAOCD*** *Traditional Rotating Intern, PGY-1, Largo Medical Center, Largo, FL **Dermatology Resident, PGY-2, Largo Medical Center, Largo, FL ***Program Director, Dermatology Residency, Largo Medical Center, Largo, FL Disclosures: None Correspondence: Anny Xiao, DO; Largo Medical Center, Graduate Medical Education, 201 14th St. SW, Largo, FL 33770; 510-684-4190; [email protected] Abstract Leprosy is a rare, chronic, granulomatous infectious disease with cutaneous and neurologic sequelae. It can be a challenging differential diagnosis in dermatology practice due to several overlapping features with rheumatologic disorders. Patients with leprosy can develop reactive states as a result of immune complex-mediated inflammatory processes, leading to the appearance of additional cutaneous lesions that may further complicate the clinical picture. We describe a case of a woman presenting with a long history of a recurrent bullous rash with chronic ulcers, with an evolution of vasculitic diagnoses, who was later determined to have lepromatous leprosy with reactive erythema nodosum leprosum (ENL). Introduction accompanied by an intense bullous purpuric rash on management of sepsis secondary to bacteremia, Leprosy is a slowly progressive disease caused by bilateral arms and face. For these complaints she was with lower-extremity cellulitis as the suspected infection with Mycobacterium leprae (M. leprae). seen in a Complex Medical Dermatology Clinic and source. A skin biopsy was taken from the left thigh, Spread continues at a steady rate in several endemic clinically diagnosed with cutaneous polyarteritis and histopathology showed epidermal ulceration countries, with more than 200,000 new cases nodosa. -

Lepromatous Leprosy Masquerading As Rhinophyma
International Journal of Otorhinolaryngology and Head and Neck Surgery Krishna S et al. Int J Otorhinolaryngol Head Neck Surg. 2015 Jul;1(1):34-36 http://www.ijorl.com pISSN 2454-5929 | eISSN 2454-5937 DOI: http://dx.doi.org/10.18203/issn.2454-5929.ijohns20150585 Case Report Lepromatous leprosy masquerading as rhinophyma 1 1 1 2 Sowmyashree Krishna *, Malcolm Pinto , Manjunath Mala Shenoy , Mahesh SG 1 Department of Dermatology, Yenepoya Medical College, Yenepoya University, Mangalore, Karnataka, India 2Department of Otolaryngology-Head and Neck Surgery, A.J. Institute of Medical Sciences and Research Center, Mangalore, Karnataka, India Received: 26 May 2015 Accepted: 24 June 2015 *Correspondence: Dr. Sowmyashree Krishna, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Leprosy a major global health problem, especially in the developing world, is an infectious disease caused by Mycobacterium leprae. Leprosy has a predilection to with cooler areas of the body. Lepromatous leprosy presents with varied manifestations like nodules, cervical lymphadenitis, hyperpigmented patches and other presentations which can mimic various other diseases and pose a diagnostic challenge in endemic areas. We report a case presenting with nodular infiltration of the nose mimicking rhinophyma who presented with faint reddish swelling over the nose which progressed to nodular infiltration. There was bilateral symmetrical thickening of nerves following which diagnosis was confirmed by slit skin smear and the patient was started on multibacillary multidrug therapy. -

Toman's Tuberculosis Case Detection, Treatment, and Monitoring
TOMAN’S TUBERCULOSIS TOMAN’S TUBERCULOSIS CASE DETECTION, TREATMENT, AND MONITORING The second edition of this practical, authoritative reference book provides a rational basis for the diagnosis and management of tuberculosis. Written by a number of experts in the field, it remains faithful to Kurt Toman’s original question-and-answer format, with subject matter grouped under the three headings Case detection, Treatment, and Monitoring. It is a testament to the enduring nature of the first edition that so much CASE DETECTION, TREA material has been retained unchanged. At the same time, the new edition has had not only to address the huge resurgence of tuber- culosis, the emergence of multidrug-resistant bacilli, and the special needs of HIV-infected individuals with tuberculosis, but also to encompass significant scientific advances. These changes in the profile of the disease and in approaches to management have inevitably prompted many new questions and answers and given a different complexion to others. Toman’s Tuberculosis remains essential reading for all who need to AND MONITORING TMENT, QUESTIONS learn more about every aspect of tuberculosis – case-finding, manage- ment, and effective control strategies. It provides invaluable support AND to anyone in the front line of the battle against this disease, from ANSWERS programme managers to policy-makers and from medical personnel to volunteer health workers. SECOND EDITION ISBN 92 4 154603 4 WORLD HEALTH ORGANIZATION WHO GENEVA Toman’s Tuberculosis Case detection, treatment, and monitoring – questions and answers SECOND EDITION Edited by T. Frieden WORLD HEALTH ORGANIZATION GENEVA 2004 WHO Library Cataloguing-in-Publication Data Toman’s tuberculosis case detection, treatment, and monitoring : questions and answers / edited by T. -

Drug Delivery Systems on Leprosy Therapy: Moving Towards Eradication?
pharmaceutics Review Drug Delivery Systems on Leprosy Therapy: Moving Towards Eradication? Luíse L. Chaves 1,2,*, Yuri Patriota 2, José L. Soares-Sobrinho 2 , Alexandre C. C. Vieira 1,3, Sofia A. Costa Lima 1,4 and Salette Reis 1,* 1 Laboratório Associado para a Química Verde, Rede de Química e Tecnologia, Departamento de Ciências Químicas, Faculdade de Farmácia, Universidade do Porto, 4050-313 Porto, Portugal; [email protected] (A.C.C.V.); slima@ff.up.pt (S.A.C.L.) 2 Núcleo de Controle de Qualidade de Medicamentos e Correlatos, Universidade Federal de Pernambuco, Recife 50740-521, Brazil; [email protected] (Y.P.); [email protected] (J.L.S.-S.) 3 Laboratório de Tecnologia dos Medicamentos, Universidade Federal de Pernambuco, Recife 50740-521, Brazil 4 Cooperativa de Ensino Superior Politécnico e Universitário, Instituto Universitário de Ciências da Saúde, 4585-116 Gandra, Portugal * Correspondence: [email protected] (L.L.C.); shreis@ff.up.pt (S.R.) Received: 30 October 2020; Accepted: 4 December 2020; Published: 11 December 2020 Abstract: Leprosy disease remains an important public health issue as it is still endemic in several countries. Mycobacterium leprae, the causative agent of leprosy, presents tropism for cells of the reticuloendothelial and peripheral nervous system. Current multidrug therapy consists of clofazimine, dapsone and rifampicin. Despite significant improvements in leprosy treatment, in most programs, successful completion of the therapy is still sub-optimal. Drug resistance has emerged in some countries. This review discusses the status of leprosy disease worldwide, providing information regarding infectious agents, clinical manifestations, diagnosis, actual treatment and future perspectives and strategies on targets for an efficient targeted delivery therapy. -

Commuin ICABLE DI SEASES STUDENT TEXT 1980
COMMUiN I CABLE DI SEASES STUDENT TEXT 1980 Rural Health Development Project Ministry of Health and Social Welfare Maseru, Lesotho ACK NOWLE;DGEMEN:'TS Nurse C.inician tVaini.nq mateL ial :;are Lesotho adaptations based upon the ME:DiEX proLotype curriculum for L'a.inin mid-Lo vol health workers. ['le prototype MiDEX matLerials 'or developed by Lhe Halth Manpowe r DovelO\opient Sta :ff of the ,Iohn A.Itirls School Med f iie, Univrsity of Iawai . The or.'ig.nili .1 prototypeS we re based on ttraini.nq ex2.U, IiiOn Ce in over a dozen third-world ccuntrios. These were reviaed on the basis of MDS experienaace in Micronesia, Till.and, Pakistan, and Guy ana beftore being made availab.Le to Lesotho under ai UI.S.A.I.D. funded 'ontract. Major adaptation in lesotho began at: the National Nurse Clini~cian T'ira. ninq " ,oqraimmo Curr iculum Adaptation Works:l'.hopt ld a, , 'Mzv.od in ,.nuary L98G. The ncar.y Li fty parti2i.paniLa uce senLtd alI majcr halth and ;i'ualth related ativ iuLits in Lesotho, hoth G ove rnienL and prIvate. h'iie'e participants and othrs workinj as irdividuas and tLhen as rvrev, i commi tees have adapted the Nurse Cli.niciai traini 1 aterLj.sL to eeLt the conditions and nee:ds of Lesoatho. The 6overnment of lenotho and particularly the staff of the Nurse C linir'i.an traini.ing 'rogrmme are grateful to IlMDS for :supilyin, the proottype materials and to a].]. thos individuals h.;Io have nelped in the Lesotho adaptation ioI. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

Supplementary Material a Gene Expression Signature Associated
Supplementary Material A Gene Expression Signature Associated With Overall Survival in Patients With Hepatocellular Carcinoma Suggests a New Treatment Strategy Jean-Pierre Gillet, Jesper B. Andersen, James P. Madigan, Sudhir Varma, Rachel K. Bagni, Katie Powell, William E. Burgan, Chung-Pu Wu, Anna Maria Calcagno, Suresh V. Ambudkar, Snorri S. Thorgeirsson, Michael M. Gottesman Journal: Molecular Pharmacology Table S1: Compounds highlighted through the Connectivity Map tool Rank for Name of drug on Connectivity Map score - Number of Connectivity Map Probability of getting Proportion of other drugs Drug signature is drug, rated Connectivity Map similarity between the experiments used score normalized an enrichment this that share this same significant for what according to gene expression signature to compute mean using scores from high if there is no signature (smaller values percent of the "n" p-value of drug and the signature similarity random selections of connection between mean that the signature is experiments? used as input genes drug signature and very specific to this input signature particular drug) Rank C-map name Mean n Enrichment p Specificity Percent non-null 1 8-azaguanine 0.916 4 0.975 0 0 100 2 adiphenine -0.765 5 -0.909 0 0.0242 100 3 trichostatin A 0.485 182 0.306 0 0.7204 73 4 tanespimycin 0.474 62 0.297 0 0.3834 72 5 apigenin 0.851 4 0.937 0.00002 0.0234 100 6 0175029-0000 0.797 6 0.86 0.00002 0.0177 100 7 thiamphenicol -0.711 5 -0.903 0.00004 0 100 8 thioguanosine 0.821 4 0.902 0.0001 0.0177 100 9 viomycin -0.723 -

RIFAMPIN (Veterinary—Systemic)
RIFAMPIN (Veterinary—Systemic) Some commonly used brand names for human-labeled products are: with erythromycin and rifampin has been reported.{R-39} Cross- Rifadin; Rifadin IV; Rimactane; and Rofact. resistance to other antibiotics or transfer of resistance to other local microorganisms has not been reported.{R-2; 4} Category: Antibacterial (systemic). Accepted ELUS,CAN EL Pneumonia, Rhodococcus equi (treatment adjunct) ; or Indications ELUS,CANExtrapulmonary infection, Rhodococcus equi (treatment Note: Rifampin is not specifically approved for veterinary use. In adjunct)EL—Foals: Rifampin is used in combination with ELUS ELCAN other USP information monographs the and erythromycin in the treatment of pneumonia caused by designations refer to uses that are not included in U.S. and Rhodococcus (Corynebacterium) equi infection in foals.{R-33; 34; 36} Canadian product labeling; however, in this monograph they Although the lung appears to be most vulnerable to Rhodococcus reflect the lack of veterinary products and, therefore, product equi infection, in some cases susceptible foals have been found to labeling. have abdominal or subcutaneous abscesses, bacterial endocarditis, diskospondylitis, gastrointestinal infections, osteomyelitis, or General considerations septicemia.{R-37-42} In many, but not all of these cases, the foal has Rifampin is a broad-spectrum antibiotic, with activity against many an accompanying pneumonia.{R-37-42} gram-positive and some gram-negative aerobic bacteria as well as R. equi are susceptible in vitro to erythromycin alone, and facultative anaerobic organisms.{R-7; 53; 60} However, for clinical erythromycin alone has been effective in the treatment of this purposes, rifampin generally should not be considered broad- infection.{R-36; 66; 67; 75} However, no studies have been performed spectrum until proven so in each case.