Paula Nathaly Torres Peña
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Memoria Técnica Distrito Metropolitano De Quito
MEMORIA TÉCNICA DISTRITO METROPOLITANO DE QUITO PROYECTO: “GENERACIÓN DE GEOINFORMACIÓN PARA LA GESTIÓN DEL TERRITORIO A NIVEL NACIONAL, ESCALA 1:25.000” INFRAESTRUCTURA Y SERVICIOS Diciembre 2013 Distrito Metropolitano de Quito Infraestructura y Servicios AGRADECIMIENTOS El conjunto de resultados y procedimientos presentados en la memoria técnica del Distrito Metropolitano de Quito representa el esfuerzo y la colaboración de instituciones como SENPLADES, GADPP, MAGAP, INEC y SIGTIERRAS, que de una u otra manera aportaron para la realización de los mismos, por cuanto contribuyen en la ejecución de actividades relacionadas con la planificación y la toma de decisiones o formulación de las propuestas de los Planes de Desarrollo y Ordenamiento Territorial. En este sentido, expresamos nuestro agradecimiento a la Secretaría Nacional de planificación y Desarrollo –SENPLADES- por el financiamiento otorgado para la realización del proyecto a nivel nacional. De igual forma, agradecemos al Gobierno Autónomo Descentralizado de la Provincia de Pichincha, que en el marco de sus competencias e implementación de políticas públicas dentro de su circunscripción territorial, ha colaborado mediante la dotación de insumos e infraestructura necesarios para la ejecución del proyecto en la provincia de Pichincha. 2 Distrito Metropolitano de Quito Infraestructura y Servicios PERSONAL PARTICIPANTE El desarrollo de ésta propuesta metodológica, demandó la participación de funcionarios del Instituto Espacial Ecuatoriano, para el proyecto de “Generación de Geoinformación Para La Gestión Del Territorio A Nivel Nacional, Escala 1:25.000”; profesionales con amplia experiencia y conocimiento en cartografía, catastro, sistemas de información geográfica, posicionamiento global, entre otros. Funcionarios del IEE: Ing. Diego Montoya Ing. Vianka Vizuete Ing. Benjamín Velateguí Top. -

Libro Kitu Territorio Solar En La Mitad Del Tiempo.Pdf
Kitu territorio solar en la mitad del tiempo DIRECCIÓN DE GESTIÓN DE CULTURA Y DEPORTES DIRECCIÓN DE GESTIÓN DE COMUNICACIÓN Kitu territorio solar en la mitad del tiempo Gobierno de la Provincia de Pichincha Edición: Mónica León Arroba Corrección de texto: Verónica Jarrín Machuca Diseño y diagramación: Ernesto Proaño Vinueza Fotografía página 76 Imagen 1con permiso de Museo La Catedral Fotografía página 84 Imagen 7 con permiso Convento San Agustín Quito, Ecuador 2012 Kitu territorio solar en la mitad del tiempo Hólguer Jara Chávez Alfonso Ortiz Crespo Alfredo Santillán Cornejo CONTENIDO PRESENTACIÓN María Pilar Vela 9 INTRODUCCIÓN Mónica León Arroba 11 KITU, ANTES DE SAN FRANCISCO DE QUITO El ‘inticentrismo’ prehispánico y la búsqueda de evidencias arqueológicas en el territorio kitu Hólguer Jara Chávez 19 DE SOLES, LUNAS Y ESTRELLAS Aproximación, ni astronómica ni mágica, a algunos elementos presentes en una portada de la Catedral Primada de Quito Alfonso Ortiz Crespo 69 QUITO: METRÓPOLI IMAGINADA Y DIVERSIDADES EN TENSIÓN Alfredo Santillán Cornejo 107 7 PRESENTACIÓN Con la finalidad de generar espacios de diálogo desde las distintas visiones, y en la comprensión de un conocimiento que debe irse construyendo desde las diversidades con la interrelación de las vivencias cotidianas, los saberes populares y la academia, la Dirección de Gestión de Cultura y Deportes del GADPP ha impulsado una serie de actividades encaminadas a motivar la reflexión, el análisis y la difusión de las distintas comprensiones históricas, antropológicas, etnográficas, arqueológicas, literarias, y demás expresiones científicas relacionadas con las culturas e identidades presentes en la Provincia de Pichincha y en el territorio nacional. -

ORDENACIÓN DEL TERRITORIO Y CONCEPCIÓN SIMBÓLICA DEL ESPACIO EN LA CIUDAD PREHISPANA DE QUITO Dr
ORDENACIÓN DEL TERRITORIO Y CONCEPCIÓN SIMBÓLICA DEL ESPACIO EN LA CIUDAD PREHISPANA DE QUITO Dr. Arquitecto Alfredo Lozano Castro 144 QUITO PREHISPÁNICO 1. INTRODUCCIÓN os estudios sobre las ciudades prehispanas en la región andi- 1 na , que viene realizando el autor, y en particular el de la ciudad de Quito, en la zona ecuatorial, han permitido fundamentar la L tesis que: “Quito, es una ciudad planificada en orden a los prin- cipios y conocimientos de las ciencias del territorio y concep- ción simbólica del espacio, conocidas por las culturas indígenas: Quito (Kitu), Cara (Kara), e Inka”. Dichos conocimientos que al parecer, desde épocas inmemoriales, poseían las culturas nativas, se dedujeron de la observación de los fenómenos estelares y del movimiento aparente de las figuras celestes, de los cuales inventaron los métodos e instrumentos para señalar la orientación y localización de los centros poblados y sus respectivos hitos geográficos, así como, la forma de sus trazados, como tendremos ocasión de demostrar en este trabajo. 1 Los significados urbanos y arqui- En efecto, la investigación realizada sobre la ciudad y entorno circundante de Quito, para tectónicos en la encontrar las huellas de su trazado aborigen, ha permitido comprobar, que existe una íntima evolución de la ciudad hispano- relación y complementariedad entre lo que el autor denomina, las ciencias del territorio y la andina. Caso del Ecuador. concepción simbólica del espacio, cuyos fundamentos se exponen a continuación. En primer Tésis doctoral, 2 E.T.S. de lugar, las ciencias del territorio , aluden al concurso de la razón, y por consiguiente a la siste- Arquitectura matización e invención de técnicas e instrumentos que permiten la intervención en el territo- Universidad Politécnica de rio, de ello derivan los sistemas de: observación estelar (ángulos intersolsticial, registro de figu- Madrid. -

Meettrrooppoolliittaann
Cooperación Científica y Técnica entre el Municipio del Distrito Metropolitano de Quito (MDMQ) y el Institut de Recherche pour le Développement (IRD) VVVAAALLLOOORRR DDDEEELLL SSSUUUEEELLLOOO EEENNN EEELLL DDDIIISSSTTTRRRIIITTTOOO MMMEEETTTRRROOOPPPOOOLLLIIITTTAAANNNOOO DDDEEE QQQUUUIIITTTOOO Cartografía, análisis, metadatos Autores: Alex Tupiza Robert D’Ercole Coordinadores del programa de Investigación Robert D’Ercole Pascale Metzger Quito, septiembre del 2001 INDICE INTRODUCCIÓN 1. VALOR DEL SUELO EN EL DMQ: ANTECEDENTES 1.1 – Estudio de 1975 1.2 – Estudio de 1990 1.3 – Estudio del 1995 1.4 – Estudio del 1997 1.4.1. Sector económico 1 1.4.2. Sector económico 2 1.4.3. Sector económico 3 1.4.4. Sector económico 4 1.4.5. Sector económico 5 1.4.6. Sector económico 6 1.4.7. Sector económico 7 1.4.8. Sector económico 8 1.4.9. Sector económico 9 1.5 – Necesidad de actualizar 2. VALOR DEL SUELO EN EL DMQ EN EL 2000 2.1. Metodología 2.1.1. Zonificación y construcción 2.1.2. Infraestructura 2.1.3. Valores del mercado 2.1.4. Clasificación de los sectores económicos 2.1.4.1. Valoración comercial 2.1.4.2. Valoración económica (sintética) 2.2. Análisis de la repartición geográfica de los sectores económicos en el DMQ 2.2.1. Sector económico 1 2.2.2. Sector económico 2 2.2.3. Sector económico 3 2.2.4. Sector económico 4 2.2.5. Sector económico 5 2.2.6. Sector económico 6 2.2.7. Sectores económicos 7, 8 y 9 2.3. Análisis de la repartición geográfica del valor comercial del suelo en el DMQ 2.3.1. -

Electoral Observation in Ecuador 1998
Electoral Observations in the Americas Series, No. 17 Electoral Observation in Ecuador 1998 Secretary General César Gaviria Assistant Secretary General Christopher R. Thomas Executive Coordinator, Unit for the Promotion of Democracy Elizabeth M. Spehar This publication is part of a series of UPD publications of the General Secretariat of the Organization of American States. The ideas, thoughts, and opinions expressed are not necessarily those of the OAS or its member states. The opinions expressed are the responsibility of the authors. OEA/Ser.D/XX SG/UPD/II. 17 23 March, 1999 Original: Spanish Electoral Observation in Ecuador 1998 General Secretariat Organization of American States Washington, D.C. 20006 1999 This report was produced under the technical supervision of Edgardo Costa Reis, Chief of Mission and Specialist of the Unit for the Promotion of Democracy (UPD). Design and composition of this publication was done by the Information and Dialogue Section of the UPD, headed by Caroline Murfitt-Eller. Betty Robinson helped with the editorial review of this report and, Meghan Henderson and Esther Rodriguez with its production. Copyright Ó 1999 by OAS. All rights reserved. This publication may be reproduced provided credit is given to the source. Contents Foreword ................................................................................................................................... vii Introduction ................................................................................................................................ix -

Municipio Del Distrito Metropolitano De Quito Secretaría General De Planificación
MUNICIPIO DEL DISTRITO METROPOLITANO DE QUITO SECRETARÍA GENERAL DE PLANIFICACIÓN PLAN OPERATIVO ANUAL - POA / EJERCICIO FISCAL 2011 DEPENDENCIA DEL MDMQ EMPRESA PÚBLICA METROPOLITANA DE OBRAS PUBLICAS Y MOVILIDAD ESTUDIO / Fecha de OBRA / Medida de Medida con valoración Unidad de Adm. Zonal Ubicación MONTO DE entrega Inicio de Fin de AVANCE Reporta (Gerencia) PROYECTO DE NOMBRE DEL PROYECTO valoración Total avance planificado Barrio Estado Medida responsable Parroquia INVERSIÓN 2011 de Obra Obra FISICO GESTION / de la obra ene- dic/2011 estudios ADQUISICION Asistencia Social,Pueblo Unido,Lucha de los Pobres,Argelia Alta,Oriente Quiteño,San Bartolo Alto,Aida Quitumbe, La Argelia, Mejoramiento Av. Simón Bolívar Tramos III km Quitumbe,Eloy León,Vertientes Unidas,Forestal Alta,San GOP Obra 13.50 9.40 La Ferroviaría, 15,279,285.25 04-Nov-10 25/05/11 04/04/12 E 35% (Autopista Rumiñahui - Av. Maldonado) (Repavimentación) Alfaro,Manuela Sáenz Patricio de Puengasí,Luluncoto Alto,San Puengasí Isidro de Puengasí,Edén del Valle,Vista Hermosa,Patrimonio Familiar,1ero de Mayo Alma Lojana,Triángulo de Mejoramiento Av. Simón Bolívar Tramos II km Manuela GOP Obra 10.60 2.07 Puengasí,Cumbayá Piedra,Collacoto,Auqui,Urbanización La 13,405,539.10 04-Nov-10 21/10/11 20/10/12 P (Vía Interoceánica - Autopista Rumiñahui) (Repavimentación) Sáenz,Tumbaco Mira,Monte Olivo Mejoramiento Av. Simón Bolívar Tramos I km Tumbaco,Eugenio Miravalle,Parque Metropolitano,Las GOP Obra 5.40 NA Cumbayá,Nayón - 04/07/11 30/01/12 26/07/12 P (Vía Interoceánica - Redondel Nayón) (Repavimentación) Espejo Palmas Mejoramiento Av. Mariscal Sucre, Tramo u (Número de GOP Obra Norte: Intersección Plaza de Los 1.00 NA La Delicia El Condado,Ponceano Los Cipreses 5,000,000.00 16/09/11 16/01/12 18/01/13 P intercambiadores) Adolescentes. -

Anexo Informe
SECRETARÍA GENERAL DE COORDINACIÓN TERRITORIAL Y PARTICIPACIÓN CIUDANA INFORME DE PARTICIPACIÓN CIUDADANA DEL PROCESO DE DELIMITACIÓN DE BARRIOS Y SECTORES DEL DISTRITRO METROPOLITANO DE QUITO SEPTIEMBRE 2020 Contenido 1. ANTECEDENTES ..................................................................................................................... 2 2. OBJETIVOS DE PARTICIPACIÓN CIUDADANA EN EL PROCESO PARA LA DELIMITACIÓN DE LOS BARRIOS Y SECTORES DEL DISTRITO METROPOLITANO DE QUITO ..................................... 2 3. ALCANCE Y POBLACIÓN OBJETIVO ....................................................................................... 3 4. MARCO LEGAL ....................................................................................................................... 4 5. METODOLOGÍA DE PARTICIPACIÓN CIUDADANA ............................................................... 5 Reuniones presenciales ..................................................................................................... 5 6. RESULTADOS DEL PROCESO DE PARTICIPIACIÓN CIUDADANA .......................................... 5 RESULTADOS DEL PROCESO DE PARTICIPACIÓN CIUDADANA ................................................... 6 PRINCIPLAES OBSERVACIONES DETECTADAS EN EL TERRITORIO Y SUS ACUERDOS ALCANZADOS EN LA PARTICIPACIÓN CIUDADANA ..................................................................... 8 CONCLUSIONES ........................................................................................................................... 21 RECOMENDACIONES -

1 TESIS DAVID CLERQUE VÁSQUEZ.Pdf
PONTIFICIA UNIVERSIDAD CATÓLICA DEL ECUADOR SEDE IBARRA ESCUELA DE CIENCIAS AGRÍCOLAS Y AMBIENTALES INFORME FINAL DEL PROYECTO TEMA: “ESTABLECIMIENTO DE UN PLAN DE CONTINGENCIA BASADO EN MEDICIONES DE PLUVIOSIDAD DEL RADAR METEOROLÓGICO DE MONJAS PARA EL DISTRITO METROPOLITANO DE QUITO”. PREVIO A LA OBTENCIÓN DEL TÍTULO DE INGENIERO EN CIENCIAS AMBIENTALES Y ECODESARROLLO LÍNEAS DE INVESTIGACIÓN: 2. Ambiente y Biodiversidad 2.2. Impactos Ambientales AUTOR: David Clerque Vásquez ASESOR: Ph D César Zuleta IBARRA, ABRIL – 2018 Ibarra, 26 de marzo del 2018 Ph D. César Zuleta ASESOR CERTIFICA: Haber revisado el presente informe final de investigación, el mismo que se ajusta a las normas vigentes en la Escuela de Ciencias Agrícolas y Ambientales (ECAA) de la Pontificia Universidad Católica del Ecuador Sede Ibarra (PUCESI); en consecuencia, autorizo su presentación para los fines legales pertinentes. (f:) ………………………………… Ph D César Alonso Zuleta Padilla C.C.: 1001037546 i PÁGINA DE APROBACIÓN DEL TRIBUNAL El jurado examinador, aprueba el presente informe de investigación en nombre de la Pontificia Universidad Católica del Ecuador Sede Ibarra (PUCESI): (f): …………………………………. Ph D. César Alonso Zuleta Padilla C.C.: 1001037546 (f): …………………………………. Mgs. Edmundo René Recalde Posso C.C.: 1001774494 (f): …………………………………. Mgs. José Valdemar Andrade Cadena C.C.: 1001927167 ii ACTA DE CESIÓN DE DERECHOS Yo David Alejandro Clerque Vásquez, declaro conocer y aceptar la disposición del Art. 165 del Código Orgánico de la Economía Social de los Conocimientos, Creatividad e Innovación, que manifiesta textualmente: “Se reconoce facultad de los autores y demás titulares de derechos de disponer de sus derechos o autorizar las utilizaciones de sus obras o prestaciones, a título gratuito u oneroso, según las condiciones que determinen. -

Tecnológico De Turismo Y Hotelería Internacional Ithi
TECNOLÓGICO DE TURISMO Y HOTELERÍA INTERNACIONAL ITHI TESIS DE GRADO PREVIA A LA OBTENCIÓN DEL TÍTULO DE TECNÓLOGO GUÍA NATURALISTA TEMA: La inserción de Guápulo en el ámbito turístico a través de un full day con el Centro Histórico. AUTOR: ALEX MARCELO ARMIJOS JARAMILLO DIRECTOR DE TESIS FELIX PATRICIO MONCAYO QUITO – ECUADOR 30 DE MAYO 2000 1 CARTA DE RESPONSABILIDAD DEL (LOS) TUTORES CERTIFICO Haber revisado el presente informe de investigación, que se ajusta a las normas institucionales y académicas establecidas por el Instituto Tecnológico de Turismo y Hotelería ITHI, de Quito; por lo tanto, se autoriza su presentación final para los fines legales pertinentes. _____________________________ Félix Patricio Moncayo Quito, 30 de Mayo 2008 2 CARTA DE RESPONSABILIDAD DEL (LOS AUTORES DEL INFORME) AUTORÍA Yo Alex Marcelo Armijos Jaramillo autor del presente informe, me responsabilizo por los conceptos, opiniones y propuestas contenidos en el mismo. Atentamente, ________________________ Alex Marcelo Armijos Jaramillo 3 ACTA DE CESIÓN DE DERECHOS DE TESIS DE GRADO Conste por el presente documento la cesión de los derechos en Tesis de Grado, de conformidad con las siguientes cláusulas: PRIMERA: El señor Patricio Moncayo, por sus propios derechos, en calidad de Tutor de Tesis; y el señor Alex Marcelo Armijos Jaramillo por sus propios derechos, en calidad de autor de la Tesis. SEGUNDA: El señor Alex Marcelo Armijos Jaramillo realizó la Tesis Titulada: “LA INSERCIÓN DE GUÁPULO EN EL ÁMBITO TURÍSTICO A TRAVÉS DE UN FULL DAY CON EL CENTRO HISTÓRICO”, para optar por el título de Tecnólogo en Guía Naturalista del Ecuador, en el Instituto Tecnológico de Turismo y Hotelería ITHI bajo la tutoría del señor Lic. -

Sedeszona9.Pdf
MINISTERIO DE EDUCACIÓN SUBSECRETARÍA DE APOYO, SEGUIMIENTO Y REGULACIÓN DE LA EDUCACIÓN PROCESO DE ASIGNACIÓN DE ESTUDIANTES A INSTITUCIONES EDUCATIVAS RÉGIMEN SIERRA 2014-2015 LISTADO DE SEDES PARA EL PROCESO DE ASIGNACIÓN DE ESTUDIANTES A INSTITUCIONES EDUCATIVAS DISTRITO* ZONA PROVINCIA CANTÓN PARROQUIA CIRCUITO NOMBRE DE LA SEDE DIRECCIÓN DE LA SEDE (Código - Nombre) 9 PICHINCHA QUITO LLANO CHICO 17D02 - CALDERON 17D02C01_06_09 ESCUELA BRETEN CALLE GARCÍA MORENO SECTOR LLANO GRANDE 9 PICHINCHA QUITO GUAYLLABAMBA 17D02 - CALDERON 17D02C10 COLEGIO GUAYLLABAMBA AV. SIMÓN BOLÍVAR, BARRIO LA MERCED N 216 CALDERÓN - UNIDAD EDUCATIVA LUXEMBURGO - HELENA 9 PICHINCHA QUITO 17D02 - CALDERON 17D02C03_04_05_07_08 RUMIÑAHUI OE11 251 E ISIDRO AYORA CARAPUNGO CORTES CALDERÓN - 9 PICHINCHA QUITO 17D02 - CALDERON 17D02C02 ESCUELA TARQUI ADELA BEDOYA Y LIZARDO BECERRA CARAPUNGO CALDERÓN - JOSÉ MIGUEL GUARDERAS Y FAUSTINO CARRASCO PARQUE 9 PICHINCHA QUITO 17D02 - CALDERON 17D02C02 COLEGIO ABDÓN CALDERÓN CARAPUNGO CENTRAL DE CALDERÓN CALDERÓN - 9 PICHINCHA QUITO 17D02 - CALDERON 17D02C03_04_05_07_08 COLEGIO NICOLÁS JIMENEZ GEOVANNY CALLES Y TOBÍAS GODOY CARAPUNGO 9 PICHINCHA QUITO COMITÉ DEL PUEBLO 17D03 - LA DELICIA 17D03C18_19 MANUEL BENJAMÍN CARRIÓN MORA ADOLFO KLINGER Y JORGE ENRIQUE GARCES 9 PICHINCHA QUITO POMASQUI 17D03 - LA DELICIA 17D03C14_15 ESCUELA QUITEÑO LIBRE AV. MANUEL CÓRDOVA GALARZA Y SANTA TERESA 9 PICHINCHA QUITO PONCEANO 17D03 - LA DELICIA 17D03C08_09_13 ESCUELA SIXTO DURÁN JOSÉ DE SOTO AV. LA PRENSA ABEL MENDEZ FRANCISCO BURGEÑO -

La Planificación Del Desarrollo Territorial En El Distrito Metropolitano De Quito
LA PLANIFICACIÓN DEL DESARROLLO TERRITORIAL EN EL DISTRITO METROPOLITANO DE QUITO LA PLANIFICACIÓN DEL DESARROLLO TERRITORIAL EN EL DISTRITO METROPOLITANO DE QUITO Municipio del Distrito Metropolitano de Quito DIRECCIÓN METROPOLITANA DE PLANIFICACIÓN TERRITORIAL Paco Moncayo Gallegos ALCALDE METROPOLITANO DE QUITO 2002-2008 Andrés Vallejo Arcos ALCALDE METROPOLITANO DE QUITO 2009 Diego Carrión Mena SECRETARIO DE DESARROLLO TERRITORIAL Juan Neira Carrasco GERENTE DE LA EMPRESA METROPOLITANA DE ALCANTARILLADO Y AGUA POTABLE Edgar Orellana Arévalo DIRECTOR EJECUTIVO DEL PROGRAMA DE SANEAMIENTO AMBIENTAL-PSA (Octubre 2002 a Julio 2008) Othón Zevallos Moreno DIRECTOR EJECUTIVO DEL PROGRAMA DE SANEAMIENTO AMBIENTAL-PSA (Agosto 2008 a la fecha) Rene Vallejo Aguirre DIRECTOR METROPOLITANO DE PLANIFICACIÓN TERRITORIAL Y SERVICIOS PÚBLICOS © Municipio del Distrito Metropolitano de Quito Secretaría de Gestión Territorial García Moreno N57 y Sucre. Quito-Ecuador Telf.: 2281 126/2281-994/ 2286-364. Fax: 2580-813 Con el apoyo técnico y financiamiento de la EMAAPQ Programa de Saneamiento Ambiental (PSA) Préstamo BID 1802-OC-EC Julio 2009 Director de diseño: Rómulo Moya Peralta Arte: Meliza de Naranjo, Amelia Molina, José Escalante Diseño, realización y preimpresión: TRAMA DISEÑO TRAMA: Juan de Dios Martínez N34-367 y Portugal. Quito-Ecuador Telfs.: (593 2) 2 246 315 / 2 922 271. http://www.trama.ec / [email protected] ÍNDICE INTRODUCCIÓN 7 1 12 EL PLAN GENERAL DE DESARROLLO TERRITORIAL 2001-2020 15 1 LA PLANIFICACIÓN Y LOS DESAFÍOS PARA EL DISTRITO METROPOLITANO -
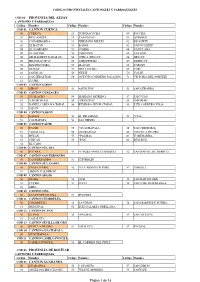
Anexo Con Codificación
CODIGOS PROVINCIALES CANTONALES Y PARROQUIALES CÓD 01 PROVINCIA DEL AZUAY CANTONES Y PARROQUIAS Código Nombre Código Nombre Código Nombre CÓD 01 CANTON CUENCA 50 CUENCA 13 TOTORACOCHA 60 PACCHA 01 BELLAVISTA 14 YANUNCAY 61 QUINGEO 02 CAÑARIBAMBA 15 HERMANO MIGUEl 62 RICAURTE 03 EL BATAN 51 BAÑOS 63 SAN JOAQUIN 04 EL SAGRARIO 52 CUMBE 64 SANTA ANA 05 EL VECINO 53 CHAUCHA 65 SAYAUSI 06 GIL RAMIREZ DAVALOS 54 CHECA (JIDCAY) 66 SIDCAY 07 HUAYNACAPAC 55 CHIQUINTAD 67 SININCAY 08 MACHANGARA 56 LLACAO 68 TARQUI 09 MONAY 57 MOLLETURO 69 TURI 10 SAN BLAS 58 NULTI 70 VALLE 11 SAN SEBASTIAN 59 OCTAVIO CORDERO PALACIOS 71 VICTORIA DEL PORTETE 12 SUCRE COD 02 CANTON GIRON 50 GIRON 51 ASUNCION 52 SAN GERARDO COD 03 CANTON GUALACEO 50 GUALACEO 54 MARIANO MORENO 57 SAN JUAN 51 *CHORDELEG 55 *PRINCIPAL 58 ZHIDMAD 52 DANIEL CORDOVA TORAL 56 REMIGIO CRESPO TORAL 59 LUIS CORDERO VEGA 53 JADAN COD 04 CANTON NABON 50 NABON 52 EL PROGRESO 54 *OÑA 51 COCHAPATA 53 LAS NIEVES COD 05 CANTON PAUTE 50 PAUTE 55 *GUACHAPALA 59 SAN CRISTOBAL 51 *AMALUZA 56 GUARAINAG 60 *SEVILLA DE ORO 52 BULAN 57 *PALMAS 61 TOMEBAMBA 53 CHICAN 58 *PAN 62 DUG DUG 54 EL CABO COD 06 CANTON PUCARA 50 PUCARA 51 *CAMILO PONCE ENRIQUEZ 52 SAN RAFAEL DE SHARUG COD 07 CANTON SAN FERNANDO 50 SAN FERNANDO 51 CHUMBLIN COD 08 CANTON STA. ISABEL 50 SANTA ISABEL 52 *EL CARMEN DE PIJILI 53 ZHAGLLI 51 ABDON CALDERON COD 09 CANTON SIGSIG 50 SIGSIG 53 GUEL 55 SAN BARTOLOME 51 CUCHIL 54 LUDO 56 SAN JOSE DE RARANGA 52 GIMA COD 10 CANTON OÑA 50 SAN FELIPE DE OÑA 51 SUSUDEL COD 11 CANTON CHORDELEG 50 CHORDELEG 52 LA UNION 54 SAN MARTIN DE PUZHIO 51 PRINCIPAL 53 LUIS GALARZA ORELLANA COD 12 CANTON EL PAN 50 EL PAN 52 *PALMAS 53 SAN VICENTE 51 *AMALUZA COD 13 CANTON SEVILLA DE ORO 50 SEVILLA DE ORO 51 AMALUZA 52 PALMAS COD 14 CANTON GUACHAPALA 50 GUACHAPALA C0D 15 CANTON CAMILO PONCE E.