Type of the Paper (Article
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Index Seminum Et Sporarum Quae Hortus Botanicus Universitatis Biarmiensis Pro Mutua Commutatione Offert
INDEX SEMINUM ET SPORARUM QUAE HORTUS BOTANICUS UNIVERSITATIS BIARMIENSIS PRO MUTUA COMMUTATIONE OFFERT ИК Е И , я ии ии . .Г. Гя и и ии , ия Biarmiae 2017 Federal State Budgetary Educational Institution of Higher Education «Perm State University», Botanic Garden ______________________________________________________________________________________ , ! 1922 . . .. – .. , .. , .. , . .. , . : , , . 2,7 . 7 500 , , , . . , . , - . . , . . , . , . 1583 . , , , , . , , (--, 1992). ... .. Ш Index Seminum 2017 2 Federal State Budgetary Educational Institution of Higher Education «Perm State University», Botanic Garden ______________________________________________________________________________________ Dear friends of the Botanic Gardens, Dear colleagues! The Botanic Garden of Perm State National Research University was founded in 1922 on the initiative of Professor A.H. Henckel and under his supervision. Many famous botanists: P.A. Sabinin, V.I. Baranov, P.A. Henckel, E.A. Pavskiy made a great contribution to the development of the biological science in the Urals. The Botanic Garden named after Prof. A.H. Henckel is a member of the Regional Council of Botanic Gardens in the Urals and has got a status of the scientific institution with protected territory. Some -

Index Seminum Et Sporarum Quae Hortus Botanicus Universitatis Biarmiensis Pro Mutua Commutatione Offert
INDEX SEMINUM ET SPORARUM QUAE HORTUS BOTANICUS UNIVERSITATIS BIARMIENSIS PRO MUTUA COMMUTATIONE OFFERT Salix recurvigemmata A.K. Skvortsov f. variegata Schumikh., O.E. Epanch. & I.V. Belyaeva Biarmiae 2020 Federal State Autonomous Educational Institution of Higher Education «Perm State National Research University», A.G. Genkel Botanical Garden ______________________________________________________________________________________ СПИСОК СЕМЯН И СПОР, ПРЕДЛАГАЕМЫХ ДЛЯ ОБМЕНА БОТАНИЧЕСКИМ САДОМ ИМЕНИ А.Г. ГЕНКЕЛЯ ПЕРМСКОГО ГОСУДАРСТВЕННОГО НАЦИОНАЛЬНОГО ИССЛЕДОВАТЕЛЬСКОГО УНИВЕРСИТЕТА Syringa vulgaris L. ‘Красавица Москвы’ Пермь 2020 Index Seminum 2020 2 Federal State Autonomous Educational Institution of Higher Education «Perm State National Research University», A.G. Genkel Botanical Garden ______________________________________________________________________________________ Дорогие коллеги! Ботанический сад Пермского государственного национального исследовательского университета был создан в 1922 г. по инициативе и под руководством проф. А.Г. Генкеля. Здесь работали известные ученые – ботаники Д.А. Сабинин, В.И. Баранов, Е.А. Павский, внесшие своими исследованиями большой вклад в развитие биологических наук на Урале. В настоящее время Ботанический сад имени А.Г. Генкеля входит в состав регионального Совета ботанических садов Урала и Поволжья, Совет ботанических садов России, имеет статус научного учреждения и особо охраняемой природной территории. Основными научными направлениями работы являются: интродукция и акклиматизация растений, -

The Vascular Flora of Boone County, Iowa (2005-2008)
Journal of the Iowa Academy of Science: JIAS Volume 117 Number 1-4 Article 5 2010 The Vascular Flora of Boone County, Iowa (2005-2008) Jimmie D. Thompson Let us know how access to this document benefits ouy Copyright © Copyright 2011 by the Iowa Academy of Science, Inc. Follow this and additional works at: https://scholarworks.uni.edu/jias Part of the Anthropology Commons, Life Sciences Commons, Physical Sciences and Mathematics Commons, and the Science and Mathematics Education Commons Recommended Citation Thompson, Jimmie D. (2010) "The Vascular Flora of Boone County, Iowa (2005-2008)," Journal of the Iowa Academy of Science: JIAS, 117(1-4), 9-46. Available at: https://scholarworks.uni.edu/jias/vol117/iss1/5 This Research is brought to you for free and open access by the Iowa Academy of Science at UNI ScholarWorks. It has been accepted for inclusion in Journal of the Iowa Academy of Science: JIAS by an authorized editor of UNI ScholarWorks. For more information, please contact [email protected]. Jour. Iowa Acad. Sci. 117(1-4):9-46, 2010 The Vascular Flora of Boone County, Iowa (2005-2008) JIMMIE D. THOMPSON 19516 515'h Ave. Ames, Iowa 50014-9302 A vascular plant survey of Boone County, Iowa was conducted from 2005 to 2008 during which 1016 taxa (of which 761, or 75%, are native to central Iowa) were encountered (vouchered and/or observed). A search of literature and the vouchers of Iowa State University's Ada Hayden Herbarium (ISC) revealed 82 additional taxa (of which 57, or 70%, are native to Iowa), unvouchered or unobserved during the current study, as having occurred in the county. -

Ep 1 915 997 A1
(19) & (11) EP 1 915 997 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 153(4) EPC (43) Date of publication: (51) Int Cl.: 30.04.2008 Bulletin 2008/18 A61K 36/48 (2006.01) A61K 8/97 (2006.01) A61K 36/18 (2006.01) A61K 36/70 (2006.01) (2006.01) (2006.01) (21) Application number: 06780703.2 A61P 17/14 A61Q 7/00 A23L 1/30 (2006.01) (22) Date of filing: 30.06.2006 (86) International application number: PCT/JP2006/313146 (87) International publication number: WO 2007/020755 (22.02.2007 Gazette 2007/08) (84) Designated Contracting States: (72) Inventor: KOHNO, Kenji AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Hadano-shi, Kanagawa 257-0004 (JP) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI SK TR (74) Representative: Rutherford, Jodie Frank B. Dehn & Co. (30) Priority: 12.08.2005 JP 2005261312 St Bride’s House 01.02.2006 JP 2006024532 10 Salisbury Square London EC4Y 8JD (GB) (71) Applicant: KOHNO, Kenji Hadano-shi, Kanagawa 257-0004 (JP) (54) AGENT FOR HAIR GROWTH (57) The aim of this invention is to offer a novel agent essed Cynanchum bungei Decne extracts ,preferably for hair growth, which has excellent hair growth effects further comprising Longan seed and/or Longan seed ex- but not side effects. The agent for hair growth of the tracts as active ingredients. This agent for hair growth present invention is characterized by comprising a proc- has no side effects when used externally or internally, it essed semi-mature soybean and/or a processed semi- can notably improve hair growth within a short period of mature soybean extracts and at least one substance se- time; ranging from 6 to 12 weeks, can return hair to its lected from the group consisting of a processed Polygoni normal hair colour (for example from white to black) and Multiflori Radix, processed Polygoni Multiflori Radix ex- can improve the gloss of hair. -

Education Edition List For
HORTICOPIA® Professional Education Edition Name Name Abelia 'Edward Goucher' Abutilon x hybridum 'Kentish Belle' Abelia chinensis Abutilon megapotamicum Abelia x grandiflora Abutilon palmeri Abelia x grandiflora 'Francis Mason' Abutilon pictum Abelia x grandiflora 'Prostrata' Abutilon pictum 'Thompsonii' Abelia x grandiflora 'Sunrise' Abutilon theophrasti Abelia schumannii Acacia abyssinica Abelia zanderi 'Conti (Confetti™)' Acacia aneura Abelia zanderi 'Sherwoodii' Acacia auriculiformis Abeliophyllum distichum Acacia baileyana Abelmoschus esculentus Acacia baileyana 'Purpurea' Abies alba Acacia berlandieri Abies amabilis Acacia cultriformis Abies balsamea Acacia farnesiana Abies bracteata Acacia greggii Abies cephalonica Acacia longifolia Abies cilicica Acacia melanoxylon Abies concolor Acacia pendula Abies concolor 'Argentea' Acacia pravissima 'Golden Carpet' Abies firma Acacia redolens Abies fraseri Acacia salicina Abies grandis Acacia saligna Abies homolepis Acacia stenophylla Abies koreana Acacia willardiana Abies lasiocarpa Acalypha hispida Abies lasiocarpa ssp. arizonica Acalypha wilkesiana Abies lasiocarpa ssp. arizonica 'Compacta' Acanthus balcanicus Abies magnifica Acanthus mollis Abies nordmanniana Acanthus spinosus Abies procera Acca sellowiana Abutilon x hybridum Acer buergerianum HORTICOPIA® Professional Education Edition Page 1 of 65 Name Name Acer campestre Acer palmatum (Dissectum Group) 'Crimson Queen' Acer capillipes Acer palmatum (Dissectum Group) 'Inaba shidare' Acer cappadocicum Acer palmatum (Dissectum Group) 'Red -

Bulletin of the Natural History Museum
Bulletin of _ The Natural History Bfit-RSH MU8&M PRIteifTBD QENERAl LIBRARY Botany Series VOLUME 23 NUMBER 2 25 NOVEMBER 1993 The Bulletin of The Natural History Museum (formerly: Bulletin of the British Museum (Natural History)), instituted in 1949, is issued in four scientific series, Botany, Entomology, Geology (incorporating Mineralogy) and Zoology. The Botany Series is edited in the Museum's Department of Botany Keeper of Botany: Dr S. Blackmore Editor of Bulletin: Dr R. Huxley Assistant Editor: Mrs M.J. West Papers in the Bulletin are primarily the results of research carried out on the unique and ever- growing collections of the Museum, both by the scientific staff and by specialists from elsewhere who make use of the Museum's resources. Many of the papers are works of reference that will remain indispensable for years to come. All papers submitted for publication are subjected to external peer review for acceptance. A volume contains about 160 pages, made up by two numbers, published in the Spring and Autumn. Subscriptions may be placed for one or more of the series on an annual basis. Individual numbers and back numbers can be purchased and a Bulletin catalogue, by series, is available. Orders and enquiries should be sent to: Intercept Ltd. P.O. Box 716 Andover Hampshire SPIO lYG Telephone: (0264) 334748 Fax: (0264) 334058 WorW Lwr abbreviation: Bull. nat. Hist. Mus. Lond. (Bot.) © The Natural History Museum, 1993 Botany Series ISSN 0968-0446 Vol. 23, No. 2, pp. 55-177 The Natural History Museum Cromwell Road London SW7 5BD Issued 25 November 1993 Typeset by Ann Buchan (Typesetters), Middlesex Printed in Great Britain at The Alden Press. -

WUCOLS 2015 Plant List for So.Coastal Region.Xlsx
WUCOLS - South Coastal Region Type Botanical Name Common Name Water Use S Abelia chinensis Chinese abelia Unknown S Abelia floribunda Mexican abelia Moderate/Medium S Abelia mosanensis 'Fragrant Abelia' fragrant abelia Unknown S Abelia parvifolia (A. longituba) Schuman abelia Unknown Gc S Abelia x grandiflora and cvs. glossy abelia Moderate/Medium S Abeliophyllum distichum forsythia Unknown S Abelmoschus manihot (Hibiscus manihot) sunset muskmallow Unknown T Abies pinsapo Spanish fir Low T N Abies spp. (CA native and non-native) fir Moderate/Medium P N Abronia latifolia yellow sand verbena Very Low P N Abronia maritima sand verbena Very Low S N Abutilon palmeri Indian mallow Low S Abutilon pictum thompsonii variegated Chinese lantern Moderate/Medium S Abutilon vitifolium flowering maple Moderate/Medium S Abutilon x hybridum & cvs. flowering maple Moderate/Medium S T Acacia abyssinica Abyssinian acacia Inappropriate S Acacia aneura mulga Low S Acacia angustissima white ball acacia Unknown T Acacia baileyana Bailey acacia Low S T Acacia berlandieri guajillo Low S A Acacia boormanii Snowy River wattle Low T Acacia cognata (A.subporosa) bower wattle Moderate/Medium S T Acacia constricta whitethorn acacia Low S Acacia covenyi blue bush Low S T Acacia craspedocarpa leatherleaf acacia Low S Acacia cultriformis knife acacia Low T Acacia dealbata silver wattle Low T Acacia decurrens green wattle Low T Acacia erioloba camel thorn Low T Acacia farnesiana (See Vachellia farnesiana) Acacia farnesiana var. farnesiana (See T Vachellia farnesiana farnesiana) -

Botanikos Sodo Augalų Sąvadas
ŠIAULI Ų UNIVERSITETAS Asta Klimien ė, Rimanta Vainorien ė, Roberta Dubosait ė-Lepeškevi čė , Aldona Grišait ė, Vaidas Juknevi čius BOTANIKOS SODO AUGAL Ų S ĄVADAS 4 Leidinyje apibendrinama informacija apie Šiauli ų universiteto Botanikos sode sukauptas augal ų takson ų ir veisli ų kolekcijas. Augal ų s ąvadas sudarytas naudojantis A. L. Tachtadžjano sistema. Lietuviški ir lotyniški augal ų vardai apib ūdinti remiantis literat ūros s ąraše pateiktais šaltiniais. Lietuviški augal ų vardai pateikti remiantis Valstybin ės lietuvi ų kalbos komisijos patvirtintu lietuvišk ų augal ų vard ų s ąrašu. Sąvade šeimos, augal ų taksonai ir veisl ės išd ėstyti alfabeto tvarka. Viršelyje melsvasis gencijonas ( Gentiana cruciata L.). R ūšis įtraukta į Lietuvos Raudonosios knygos s ąrašus. Nuotraukos autorius fotografas Romualdas Struoga. 2 TURINYS PRATARM Ė .....................................................................................................................................4 1. EQUISETOPHYTA ......................................................................................................................6 EQUISETOPSIDA ...............................................................................................................6 2. POLYPODIOPHYTA …………………………………………………………………………. 6 POLYPODIOPSIDA …………………………………………...………………………… 6 3. PINOPHYTA ................................................................................................................................7 GINKGOOPSIDA ................................................................................................................7 -

WUCOLS List S Abelia Chinensis Chinese Abelia M ? ? M / / Copyright © UC Regents, Davis Campus
Ba Bu G Gc P Pm S Su T V N Botanical Name Common Name 1 2 3 4 5 6 Symbol Vegetation Used in Type WUCOLS List S Abelia chinensis Chinese abelia M ? ? M / / Copyright © UC Regents, Davis campus. All rights reserved. bamboo Ba S Abelia floribunda Mexican abelia M ? M M / / S Abelia mosanensis 'Fragrant Abelia' fragrant abelia ? ? ? ? ? ? bulb Bu S Abelia parvifolia (A. longituba) Schuman abelia ? ? ? M ? ? grass G groundcover GC Gc S Abelia x grandiflora and cvs. glossy abelia M M M M M / perennial* P S Abeliophyllum distichum forsythia M M ? ? ? ? palm and cycad Pm S Abelmoschus manihot (Hibiscus manihot) sunset muskmallow ? ? ? L ? ? T Abies pinsapo Spanish fir L L L / / / shrub S succulent Su T N Abies spp. (CA native and non-native) fir M M M M / / P N Abronia latifolia yellow sand verbena VL VL VL / ? ? tree T P N Abronia maritima sand verbena VL VL VL / ? ? vine V California N native S N Abutilon palmeri Indian mallow L L L L M M S Abutilon pictum thompsonii variegated Chinese lantern M H M M ? ? Sunset WUCOLS CIMIS ET Representative Number climate 0 Region zones** Cities zones* S Abutilon vitifolium flowering maple M M M / ? ? Healdsburg, Napa, North- San Jose, Salinas, Central 14, 15, 16, 17 1, 2, 3, 4, 6, 8 San Francisco, Coastal San Luis Obispo S Abutilon x hybridum & cvs. flowering maple M H M M / / 1 Auburn, Central Bakersfield, Chico, 8, 9, 14 12, 14, 15, 16 Valley Fresno, Modesto, Sacramento S T Acacia abyssinica Abyssinian acacia / ? / ? / L 2 Irvine, Los South Angeles, Santa 22, 23, 24 1, 2, 4, 6 Coastal Barbara, Ventura, -

Nursery Crop Insurance Program
2000 AND SUCCEEDING CROP YEARS FEDERAL CROP INSURANCE CORPORATION ELIGIBLE PLANT LIST AND PLANT PRICE SCHEDULE NURSERY CROP INSURANCE PROGRAM • CONNECTICUT • DELAWARE • MAINE • MARYLAND • MASSACHUSETTS • NEW HAMPSHIRE • NEW JERSEY • NEW YORK • NORTH CAROLINA • PENNSYLVANIA • RHODE ISLAND • VERMONT • VIRGINIA • WEST VIRGINIA GUIDE TO COMMERCIAL NOMENCLATURE The price for each plant and size listed in the Eligible Plant List and Plant Price Schedule is your lowest wholesale price, as determined from your wholesale catalogs or price lists submitted in accordance with the Special Provisions, not to exceed the maximum price limits included in this Schedule. CONTENTS INTRODUCTION Crop Insurance Nomenclature Format Crop Type and Optional Units Storage Keys Hardiness Zone Designations Container Insurable Hardiness Zones Field Grown Minimum Hardiness Zones Plant Size SOFTWARE AVAILABILITY System Requirements Sample Report INSURANCE PRICE CALCULATION Crop Type Base Price Tables ELIGIBLE PLANT LIST AND PLANT PRICE SCHEDULE APPENDIX A County Hardiness Zones B Storage Keys C Insurance Price Calculation Worksheet D Container Volume Calculation Worksheet The DataScape Guide to Commercial Nomenclature is used in this document by the Federal Crop Insurance Corporation (FCIC), an agency of the United States Department of Agriculture (USDA), with permission. Permission is given to use or reproduce this Eligible Plant List and Plant Price Schedule for purposes of administering the Federal Crop Insurance Corporation's Nursery Insurance program only. The DataScape -

Clusiaceae.Pdf
CLUSIACEAE (GUTTIFERAE) 藤黄科 teng huang ke Li Xiwen (李锡文 Li Hsi-wen)1, Li Jie (李捷)2; Norman K. B. Robson3, Peter F. Stevens4 Trees, shrubs, or sometimes herbs containing resin or oil in schizogenous spaces or canals and sometimes black or red glands containing hypericin or pseudohypericin. Leaves simple, entire or rarely gland-fringed, opposite or sometimes whorled, nearly always estipulate. Flowers bisexual or unisexual, regular, hypogynous, solitary or in cymes or thyrses; bracteoles often inserted just beneath calyx and then not always easily distinguishable from sepals. Sepals (2–)4 or 5(or 6), imbricate or decussate or rarely wholly united in bud, inner ones sometimes petaloid. Petals [3 or]4 or 5[or 6], free, imbricate or contorted in bud. Stamens many to rarely few (9), in [3 or]4 or 5 bundles (fascicles) that are free and antipetalous or variously connate, with filaments variously united or apparently free and then sometimes sterile (staminodes); anther dehiscence longitudinal. Staminode bundles (fasciclodes) 3–5, free and antisepalous or variously connate or absent. Ovary superior, with 2–5(–12) connate carpels, 1–12-loculed, with axile to parietal or basal placentation; ovules 1 to many on each placenta, erect to pendulous; styles 1–5[–12], free or ± united or absent; stigmas 1– 12, punctiform to peltate or, when sessile, radiate, surface papillate or smooth. Fruit a septicidal or septifragal, rarely loculicidal, capsule, berry, or drupe; seeds 1 to many, without or almost without endosperm [sometimes arillate]. About 40 genera and 1200 species: mainly in tropical regions, except Hypericum and Triadenum, which are both mainly temperate in distribu- tion; eight genera (one endemic) and 95 species (48 endemic, one introduced) in China. -
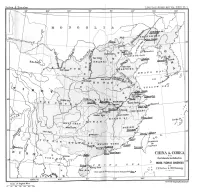
An Enumeration of All the Plants Known from China Proper, Formosa
ENUMERATION OF ALL THE PLANTS KNOWN PRON CHINA PROPER, FORMOSA, HAINAN, THE COREA, THE LUCHU BRCHIPELAGO, AND THE ISLAND OF HONGKONG; TOGETHER WITH THEIR DISTRIBUTION AND SYNONYMY. 1. RANUNCULACEB. 1. Clematis acerifolia, Mmiiit. Tl.As. Or. Xragm. p. 2 ; Kuittze, Monogr. Gatt. Clem. p. 142. CHIHLI: Poliuashan (Brefschneider !). Herb. Kew. 2. Clematis sthussfolia, Turcz. in Bull. MOSC.v. p. 181; Maxim. in Me% Biol. ix. p. 586; Sranchet, PI. David. p. 12. Clematis nutans, Royle, 6. aethusifolia, Kuntze, Monogr. Gatt. Clem. p. 129. CHIHLI:near Poking (Tatarinow! Kirilow! Bushell! Williams!); KANSUH(Przeiualski ex Maximowicz). Herb. Kew. Mandshuria. 3. dematis alpina, Mill. Diet. ed. viii. n. 9 ; DC.Prodr. i. p. 10 ; Franchet, Dacid. 11. PI. 14. \ Atragene alpina, Linn. ; Muxim. in M.41. Biol. ix. 11. 603. LIWN JOURN.-BOTANY, VOL. xxm. B 2 1. RANUNCULACEB. CHIHLI: P0huasha.n (Kirilozu! Bretschneider !) j Jehol (David ex Pranchet). Herb. Eew. Europe ; Siberia. 4. Clematis angustifolia, Jacp. Enum. p. 310, Collect. i. p. 137, et Ic. PI. Rar. I i. t. 104 ; DC. Prodr. i. p. 7 ; Naxim. in .El. Biol. ix. p. 594, et 31. As. Or. Praym. p. 2 ;~Debeaux, PI. Tche'$oou, p. 22 (var. tchefouensis) ; Banchet, PI. David. p. 14. Clematis recta, Linn., 7. angustifolia, Kuntze, Monogr. Gatt. Clem. p. 112. CHIHLI: Peking (Bushel1 ! Hancock !); SHINGEING: Talienhwaq (Xwinhoe!); Cbienshan (Ross !) ; SHANTUNG(Maingay ! Han- cock !). Mus. Brit. ; Herb. Eew. Dahuria , Mongolia, and Mandshuria. 5. Clematis apiifolia, DC Xyst. i. p. 149, kt Prodr. i. p. 6; Naxim. ill Me'l. Biol. ix. p. 593; Benth. 31. Hongk. p. 7 ; Hook.