Heat and Light Influence on Gene Expression And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stilbenes: Chemistry and Pharmacological Properties
1 Journal of Applied Pharmaceutical Research 2015, 3(4): 01-07 JOURNAL OF APPLIED PHARMACEUTICAL RESEARCH ISSN No. 2348 – 0335 www.japtronline.com STILBENES: CHEMISTRY AND PHARMACOLOGICAL PROPERTIES Chetana Roat*, Meenu Saraf Department of Microbiology & Biotechnology, University School of Sciences, Gujarat University, Ahmedabad, Gujarat 380009, India Article Information ABSTRACT: Medicinal plants are the most important source of life saving drugs for the Received: 21st September 2015 majority of the Worlds’ population. The compounds which synthesized in the plant from the Revised: 15th October 2015 secondary metabolisms are called secondary metabolites; exhibit a wide array of biological and Accepted: 29th October 2015 pharmacological properties. Stilbenes a small class of polyphenols, have recently gained the focus of a number of studies in medicine, chemistry as well as have emerged as promising Keywords molecules that potentially affect human health. Stilbenes are relatively simple compounds Stilbene; Chemistry; synthesized by plants and deriving from the phenyalanine/ polymalonate route, the last and key Structures; Biosynthesis pathway; enzyme of this pathway being stilbene synthase. Here, we review the biological significance of Pharmacological properties stilbenes in plants together with their biosynthesis pathway, its chemistry and its pharmacological significances. INTRODUCTION quantities are present in white and rosé wines, i.e. about a tenth Plants are source of several drugs of natural origin and hence of those of red wines. Among these phenolic compounds, are termed as the medicinal plants. These drugs are various trans-resveratrol, belonging to the stilbene family, is a major types of secondary metabolites produced by plants; several of active ingredient which can prevent or slow the progression of them are very important drugs. -

Desoxyrhapontigenin Inhibits RANKL‑Induced Osteoclast Formation and Prevents Inflammation‑Mediated Bone Loss
INTERNATIONAL JOURNAL OF MOleCular meDICine 42: 569-578, 2018 Desoxyrhapontigenin inhibits RANKL‑induced osteoclast formation and prevents inflammation‑mediated bone loss PHUONG THAO TRAN1, DONG-HWA PARK2, OKHWA KIM1, SEUNG-HAE KWON3, BYUNG-SUN MIN2 and JEONG-HYUNG LEE1 1Department of Biochemistry, College of Natural Sciences, Kangwon National University, Chuncheon, Gangwon-Do 24341; 2College of Pharmacy, Catholic University of Daegu, Hayang, Gyeongbuk 38430; 3Division of Bio-Imaging, Korea Basic Science Institute Chuncheon Center, Chuncheon, Gangwon-Do 24341, Republic of Korea Received November 6, 2017; Accepted March 15, 2018 DOI: 10.3892/ijmm.2018.3627 Abstract. Desoxyrhapontigenin (DRG), a stilbene compound mouse model. At the molecular level, DRG inhibited the from Rheum undulatum, has been found to exhibit various RANKL-induced activation of extracellular signal-regulated pharmacological activities, however, its impact on osteoclast kinase, the expression of c-Fos, and the induction of NFATc1, formation has not been investigated. The present study inves- a crucial transcription factor for osteoclast formation. DRG tigated the effect of DRG on receptor activator of nuclear decreased the expression levels of osteoclast marker genes, factor-κB ligand (RANKL)-induced osteoclast differen- including matrix metalloproteinase-9, tartrate-resistant acid tiation in mouse bone marrow macrophages (BMMs) and phosphatase and cathepsin K. In conclusion, these findings inflammation‑induced bone loss in vivo. BMMs or RAW264.7 suggested that DRG inhibited the differentiation of BMMs cells were treated with DRG, followed by an evaluation of into mature osteoclasts by suppressing the RANKL-induced cell viability, RANKL-induced osteoclast differentiation, activator protein-1 and NFATc1 signaling pathways, and actin-ring formation and resorption pits activity. -

Applications of Mass Spectrometry in Natural Product Drug Discovery for Malaria: Targeting Plasmodium Falciparum Thioredoxin Reductase
Applications of mass spectrometry in natural product drug discovery for malaria: Targeting Plasmodium falciparum thioredoxin reductase by Ranjith K. Munigunti A dissertation submitted to the Graduate Faculty of Auburn University in partial fulfillment of the requirements for the Degree of Doctor of Philosophy Auburn, Alabama May 5, 2013 Keywords: Chromatography, mass spectrometry, malaria, Plasmodium falciparum, thioredoxin reductase, thioredoxin Copyright 2013 by Ranjith K. Munigunti Approved by Angela I. Calderón, Chair, Assistant Professor of Pharmacal Sciences C. Randall Clark, Professor of Pharmacal Sciences Jack DeRuiter, Professor of Pharmacal Sciences Forrest Smith, Associate Professor of Pharmacal Sciences Orlando Acevedo, Associate Professor of Chemistry and Biochemistry Abstract Malaria is considered to be the dominant cause of death in low income countries especially in Africa. Malaria caused by Plasmodium falciparum is a most lethal form of the disease because of its rapid spread and the development of drug resistance. The main problem in the treatment of malaria is the emergence of drug resistant malaria parasites. Over the years/decades, natural products have been used for the treatment or prevention of number of diseases. They can serve as compounds of interest both in their natural form and as templates for synthetic modification. Nature has provided a wide variety of compounds that inspired the development of potential therapeutics such as quinine, artemisinin and lapachol as antimalarial agents. As the resistance to known antimalarials is increasing, there is a need to expand the antimalarial drug discovery efforts for new classes of molecules to combat malaria. This research work focuses on the applications of ultrafiltration, mass spectrometry and molecular modeling based approaches to identify inhibitors of Plasmodium falciparum thioredoxin reductase (PfTrxR), our main target and Plasmodium falciparum glutathione reductase (PfGR) as an alternative target for malaria drug discovery. -

Literature Review Zero Alcohol Red Wine
A 1876 LI A A U R S T T S R U A L A I A FLAVOURS, FRAGRANCES AND INGREDIENTS 6 1 7 8 7 8 1 6 A I B A L U A S R T B Essential Oils, Botanical Extracts, Cold Pressed Oils, BOTANICAL Infused Oils, Powders, Flours, Fermentations INNOVATIONS LITERATURE REVIEW HEALTH BENEFITS RED WINE ZERO ALCOHOL RED WINE RED WINE EXTRACT POWDER www.botanicalinnovations.com.au EXECUTIVE SUMMARY The term FRENCH PARADOX is used to describe the relatively low incidence of cardiovascular disease in the French population despite the high consumption of red wine. Over the past 27 years numerous clinical studies have found a linkages with the ANTIOXIDANTS in particular, the POLYPHENOLS, RESVERATROL, CATECHINS, QUERCERTIN and ANTHOCYANDINS in red wine and reduced incidences of cardiovascular disease. However, the alcohol in wine limits the benefits of wine. Studies have shown that zero alcohol red wine and red wine extract which contain the same ANTIOXIDANTS including POLYPHENOLS, RESVERATROL, CATECHINS, QUERCERTIN and ANTHOCYANDINS has the same is not more positive health benefits. The following literature review details some of the most recent positive health benefits derived from the ANTIOXIDANTS found in red wine POLYPHENOLS: RESVERATROL, CATECHINS, QUERCERTIN and ANTHOCYANDINS. The positive polyphenolic antioxidant effects of the polyphenols in red wine include: • Cardio Vascular Health Benefits • Increase antioxidants in the cardiovascular system • Assisting blood glucose control • Skin health • Bone Health • Memory • Liking blood and brain health • Benefits -
Abstract Book Rk4bw
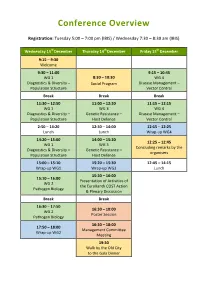
Conference Overview Registration: Tuesday 5:00 – 7:00 pm (IBIS) / Wednesday 7:30 – 8:30 am (IBIS) Wednesday 13rd December Thursday 14th December Friday 15th December 9:15 – 9:30 Welcome 9:30 – 11:00 9:15 – 10:45 WG 1 8:30 – 10:30 WG 4 Diagnostics & Diversity – Social Program Disease Management – Population Structure Vector Control Break Break Break 11:30 – 12:50 11:00 – 12:30 11:15 – 12:15 WG 1 WG 3 WG 4 Diagnostics & Diversity – Genetic Resistance – Disease Management – Population Structure Host Defence Vector Control 2:50 – 14:20 12:30 – 14:00 12:15 – 12:25 Lunch Lunch Wrap-up WG4 14:20 – 15:00 14:00 – 15:20 12:25 – 12:45 WG 1 WG 3 Concluding remarks by the Diagnostics & Diversity – Genetic Resistance – organisers Population Structure Host Defence 15:00 – 15:10 15:20 – 15:30 12:45 – 14:15 Wrap-up WG1 Wrap-up WG3 Lunch 15:30 – 16:00 15:10 – 16:00 Presentation of Activities of WG 2 the EuroXanth COST Action Pathogen Biology & Plenary Discussion Break Break 16:30 – 17:50 16:30 – 18:00 WG 2 Poster Session Pathogen Biology 16:30 – 18:00 17:50 – 18:00 Management Committee Wrap-up WG2 Meeting 19:30 Walk by the Old City to the Gala Dinner 1st Annual Conference of the EuroXanth COST Action Integrating Science on Xanthomonadaceae for integrated Plant disease management in EuroPe Organisers Joana Costa FitoLab – Instituto Pedro Nunes and University of Coimbra, Portugal Ralf Koebnik IRD, Montpellier, France Scientific Committee Jens Boch Leibniz University Hannover, Germany Vittoria Catara Wageningen University, The Netherlands Joana Costa University of Coimbra, Portugal Maria Leonor Cruz Instituto Nacional de Investigacao Agraria e Veterinaria, Oeiras, Portugal Ralf Koebnik IRD, Montpellier, France Tamas Kovacs ENVIROINVEST, Hungary Joël F. -

Biological/Chemopreventive Activity of Stilbenes and Their Effect on Colon Cancer
Review 1635 Biological/Chemopreventive Activity of Stilbenes and their Effect on Colon Cancer Author Agnes M. Rimando1, Nanjoo Suh2, 3 Affiliation 1 United States Department of Agriculture, Agricultural Research Service, Natural Products Utilization Research Unit, University, MS, USA 2 Department of Chemical Biology, Ernest Mario School of Pharmacy, Rutgers, The State University of New Jersey, Piscataway, NJ, USA 3 The Cancer Institute of New Jersey, New Brunswick, NJ, USA Key words Abstract ventive agents. One of the best-characterized ●" resveratrol ! stilbenes, resveratrol, has been known as an anti- ●" stilbenes Colon cancer is one of the leading causes of can- oxidant and an anti-aging compound as well as ●" colon cancer cer death in men and women in Western coun- an anti-inflammatory agent. Stilbenes have di- ●" inflammation tries. Epidemiological studies have linked the verse pharmacological activities, which include consumption of fruits and vegetables to a re- cancer prevention, a cholesterol-lowering effect, duced risk of colon cancer, and small fruits are enhanced insulin sensitivity, and increased life- particularly rich sources of many active phyto- span. This review summarizes results related to chemical stilbenes, such as resveratrol and pter- the potential use of various stilbenes as cancer ostilbene. Recent advances in the prevention of chemopreventive agents, their mechanisms of colon cancer have stimulated an interest in diet action, as well as their pharmacokinetics and ef- and lifestyle as an effective means of interven- ficacy for the prevention of colon cancer in ani- tion. As constituents of small fruits such as mals and humans. grapes, berries and their products, stilbenes are under intense investigation as cancer chemopre- received May 7, 2008 Introduction wood in response to fungal infection [5], [6]. -

STILBENOID CHEMISTRY from WINE and the GENUS VITIS, a REVIEW Alison D
06àutiliser-mérillonbis_05b-tomazic 27/06/12 21:23 Page57 STILBENOID CHEMISTRY FROM WINE AND THE GENUS VITIS, A REVIEW Alison D. PAWLUS, Pierre WAFFO-TÉGUO, Jonah SHAVER and Jean-Michel MÉRILLON* GESVAB (EA 3675), Université de Bordeaux, ISVV Bordeaux - Aquitaine, 210 chemin de Leysotte, CS 50008, 33882 Villenave d'Ornon cedex, France Abstract Résumé Stilbenoids are of great interest on account of their many promising Les stilbénoïdes présentent un grand intérêt en raison de leurs nombreuses biological activities, especially in regards to prevention and potential activités biologiques prometteuses, en particulier dans la prévention et le treatment of many chronic diseases associated with aging. The simple traitement de diverses maladies chroniques liées au vieillissement. Le stilbenoid monomer, -resveratrol, has received the most attention due to -resvératrol, monomère stilbénique, a suscité beaucoup d'intérêt de par E E early and biological activities in anti-aging assays. Since ses activités biologiques et . Une des principales sources in vitro in vivo in vitro in vivo , primarily in the form of wine, is a major dietary source of alimentaires en stilbénoïdes est , principalement sous forme Vitis vinifera Vitis vinifera these compounds, there is a tremendous amount of research on resveratrol de vin. De nombreux travaux de recherche ont été menés sur le resvératrol in wine and grapes. Relatively few biological studies have been performed dans le vin et le raisin. À ce jour, relativement peu d'études ont été réalisées on other stilbenoids from , primarily due to the lack of commercial sur les stilbènes du genre autre que le resvératrol, principalement en Vitis Vitis sources of many of these compounds. -

(2019- Ncov) Based on HPLC-Q-Exactive-MS/MS and Molecular Docking Method
Exploring the Active Compounds of Traditional Mongolian Medicine Agsirga in Intervention of Novel Coronavirus (2019- nCoV) Based on HPLC-Q-Exactive-MS/MS and Molecular Docking Method Jie Cheng1 ‡, Yuchen Tang1 ‡, Baoquan Bao1*, Ping Zhang1* 1 College of Pharmacy, Inner Mongolia Medical University, 010110, Hohhot, China Key words: Agsirga; 2019-nCoV; ACE2; molecular docking; HPLC- MS ABSTRACT Objective: To screen all compounds of Agsirga based on the HPLC-Q-Exactive high-resolution mass spectrometry and find potential inhibitors that can respond to 2019-nCoV from active compounds of Agsirga by molecular docking technology. Methods: HPLC-Q-Exactive high-resolution mass spectrometry was adopted to identify the complex components of Mongolian medicine Agsirga, and separated by the high-resolution mass spectrometry Q-Exactive detector. Then the Orbitrap detector was used in tandem high-resolution mass spectrometry, and the related molecular and structural formula were found by using the chemsipider database and related literature, combined with precise molecular formulas (errors ≤ 5 × 10−6) , retention time, primary mass spectra, and secondary mass spectra information, The fragmentation regularities of mass spectra of these compounds were deduced. Taking ACE2 as the receptor and deduced compounds as the ligand, all of them were pretreated by discover studio, autodock and Chem3D. The molecular docking between the active ingredients and the target protein was studied by using AutoDock molecular docking software. The interaction between ligand and receptor is applied to provide a choice for screening anti-2019-nCoV drugs. Result: Based on the fragmentation patterns of the reference compounds and consulting literature, a total of 96 major alkaloids and stilbenes were screened and identified in Agsirga by the HPLC-Q-Exactive-MS/MS method. -

Preclinical and Clinical Studies
ANTICANCER RESEARCH 24: 2783-2840 (2004) Review Role of Resveratrol in Prevention and Therapy of Cancer: Preclinical and Clinical Studies BHARAT B. AGGARWAL1, ANJANA BHARDWAJ1, RISHI S. AGGARWAL1, NAVINDRA P. SEERAM2, SHISHIR SHISHODIA1 and YASUNARI TAKADA1 1Cytokine Research Laboratory, Department of Bioimmunotherapy, The University of Texas M. D. Anderson Cancer Center, Box 143, 1515 Holcombe Boulevard, Houston, Texas 77030; 2UCLA Center for Human Nutrition, David Geffen School of Medicine, 900 Veteran Avenue, Los Angeles, CA 90095-1742, U.S.A. Abstract. Resveratrol, trans-3,5,4'-trihydroxystilbene, was first and cervical carcinoma. The growth-inhibitory effects of isolated in 1940 as a constituent of the roots of white hellebore resveratrol are mediated through cell-cycle arrest; up- (Veratrum grandiflorum O. Loes), but has since been found regulation of p21Cip1/WAF1, p53 and Bax; down-regulation of in various plants, including grapes, berries and peanuts. survivin, cyclin D1, cyclin E, Bcl-2, Bcl-xL and cIAPs; and Besides cardioprotective effects, resveratrol exhibits anticancer activation of caspases. Resveratrol has been shown to suppress properties, as suggested by its ability to suppress proliferation the activation of several transcription factors, including NF- of a wide variety of tumor cells, including lymphoid and Î B, AP-1 and Egr-1; to inhibit protein kinases including IÎ B· myeloid cancers; multiple myeloma; cancers of the breast, kinase, JNK, MAPK, Akt, PKC, PKD and casein kinase II; prostate, stomach, colon, pancreas, and thyroid; melanoma; and to down-regulate products of genes such as COX-2, head and neck squamous cell carcinoma; ovarian carcinoma; 5-LOX, VEGF, IL-1, IL-6, IL-8, AR and PSA. -
WO 2015/028324 A2 5 March 2015 (05.03.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/028324 A2 5 March 2015 (05.03.2015) P O P C T (51) International Patent Classification: Copenhagen (DK). MEYER, Jean Philippe; C/O Evolva C12N 9/10 (2006.01) SA, Duggingerstrasse 23, CH-4153 Reinach (CH). VAZQUEZ, Carlos Casado; C/O Evolva SA, Dugginger (21) International Application Number: strasse 23, CH-41 53 Reinach (CH). PCT/EP2014/067520 (74) Agent: SMAGGASGALE, Gillian, Helen; 55 Drury (22) International Filing Date: Lane, London WC2B 5SQ (GB). 15 August 2014 (15.08.2014) (81) Designated States (unless otherwise indicated, for every English (25) Filing Language: kind of national protection available): AE, AG, AL, AM, (26) Publication Language: English AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (30) Priority Data: DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, 61/872,452 30 August 2013 (30.08.2013) US HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 61/873,348 3 September 2013 (03.09.2013) US KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, 61/917,928 18 December 201 3 (18. 12.2013) US MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, 61/923,486 3 January 2014 (03.01 .2014) US OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (71) Applicant: EVOLVA SA [CH/CH]; Duggingerstrasse 23, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, CH-4153 Reinach (CH). -

Role of Resveratrol in Prevention and Therapy of Cancer: Preclinical and Clinical Studies
ANTICANCER RESEARCH 24: 2783-2840 (2004) Review Role of Resveratrol in Prevention and Therapy of Cancer: Preclinical and Clinical Studies BHARAT B. AGGARWAL1, ANJANA BHARDWAJ1, RISHI S. AGGARWAL1, NAVINDRA P. SEERAM2, SHISHIR SHISHODIA1 and YASUNARI TAKADA1 1Cytokine Research Laboratory, Department of Bioimmunotherapy, The University of Texas M. D. Anderson Cancer Center, Box 143, 1515 Holcombe Boulevard, Houston, Texas 77030; 2UCLA Center for Human Nutrition, David Geffen School of Medicine, 900 Veteran Avenue, Los Angeles, CA 90095-1742, U.S.A. Abstract. Resveratrol, trans-3,5,4'-trihydroxystilbene, was first and cervical carcinoma. The growth-inhibitory effects of isolated in 1940 as a constituent of the roots of white hellebore resveratrol are mediated through cell-cycle arrest; up- (Veratrum grandiflorum O. Loes), but has since been found regulation of p21Cip1/WAF1, p53 and Bax; down-regulation of in various plants, including grapes, berries and peanuts. survivin, cyclin D1, cyclin E, Bcl-2, Bcl-xL and cIAPs; and Besides cardioprotective effects, resveratrol exhibits anticancer activation of caspases. Resveratrol has been shown to suppress properties, as suggested by its ability to suppress proliferation the activation of several transcription factors, including NF- of a wide variety of tumor cells, including lymphoid and Î B, AP-1 and Egr-1; to inhibit protein kinases including IÎ B· myeloid cancers; multiple myeloma; cancers of the breast, kinase, JNK, MAPK, Akt, PKC, PKD and casein kinase II; prostate, stomach, colon, pancreas, and thyroid; melanoma; and to down-regulate products of genes such as COX-2, head and neck squamous cell carcinoma; ovarian carcinoma; 5-LOX, VEGF, IL-1, IL-6, IL-8, AR and PSA. -

A Reference List of Phenolic Compounds (Including Stilbenes) in Grapevine (Vitis Vinifera L.) Roots, Woods, Canes, Stems, and Leaves
antioxidants Review A Reference List of Phenolic Compounds (Including Stilbenes) in Grapevine (Vitis vinifera L.) Roots, Woods, Canes, Stems, and Leaves Piebiep Goufo 1,* , Rupesh Kumar Singh 2 and Isabel Cortez 1 1 Centre for the Research and Technology of Agro-Environment and Biological Sciences, Departamento de Agronomia, Universidade de Trás-os-Montes e Alto Douro, Quinta de Prados, 5000-801 Vila Real, Portugal; [email protected] 2 Centro de Química de Vila Real, Universidade de Trás-os-Montes e Alto Douro, Quinta de Prados, 5000-801 Vila Real, Portugal; [email protected] * Correspondence: [email protected] Received: 15 April 2020; Accepted: 5 May 2020; Published: 8 May 2020 Abstract: Due to their biological activities, both in plants and in humans, there is a great interest in finding natural sources of phenolic compounds or ways to artificially manipulate their levels. During the last decade, a significant amount of these compounds has been reported in the vegetative organs of the vine plant. In the roots, woods, canes, stems, and leaves, at least 183 phenolic compounds have been identified, including 78 stilbenes (23 monomers, 30 dimers, 8 trimers, 16 tetramers, and 1 hexamer), 15 hydroxycinnamic acids, 9 hydroxybenzoic acids, 17 flavan-3-ols (of which 9 are proanthocyanidins), 14 anthocyanins, 8 flavanones, 35 flavonols, 2 flavones, and 5 coumarins. There is great variability in the distribution of these chemicals along the vine plant, with leaves and stems/canes having flavonols (83.43% of total phenolic levels) and flavan-3-ols (61.63%) as their main compounds, respectively. In light of the pattern described from the same organs, quercetin-3-O-glucuronide, quercetin-3-O-galactoside, quercetin-3-O-glucoside, and caftaric acid are the main flavonols and hydroxycinnamic acids in the leaves; the most commonly represented flavan-3-ols and flavonols in the stems and canes are catechin, epicatechin, procyanidin B1, and quercetin-3-O-galactoside.