Volume 76, No. 3, July 2012
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Otago's Distance Learning Programme
ISSUE 9 20 May 2011 OTAGO BULLETIN FORTNIGHTLY NEWSLETTER FOR UNIVERSITY STAFF AND POSTGRADUATE STUDENTS Researchers showcase their work Photo: Sharron Bennett Photo: Participants and audience members at a public event to showcase Otago’s cutting-edge research last month. The symposium, For the Public Good, attracted a record 30 early to mid-career staff from across the four academic Divisions to the Barnett Lecture Theatre.The group volunteered to boil down their work into mere four-minute presentations, creating a series of snapshots of the exciting research under way at Otago. A member of the public audience commented afterwards that “it was better than going to the movies,” says organiser Dr Jacob Edmond, who was delighted with the turnout of researchers – double last year’s – and the extremely high standard of all the presentations. Continued on page 2... Next Research Deputy Vice-Chancellor named Otago’s next Deputy Vice-Chancellor (Research and Enterprise) in 1993. From 2002 he was the Deputy Director of the Professor Richard Blaikie is excited about returning to the MacDiarmid Institute, succeeding Sir Paul Callaghan as university at which his scientific career began. Director in 2008. Professor Blaikie, who is currently a Professor at the University In addition to his Deputy Vice-Chancellor role at Otago, of Canterbury and Director of the MacDiarmid Institute for Professor Blaikie will hold a personal Chair in Physics. Advanced Materials and Nanotechnology, will take up the He says he is looking forward to taking up his new position. position in December. He replaces Professor Harlene Hayne, whose appointment as Otago’s next Vice-Chancellor was “Otago is noted for the strength of its research and my goal is to announced earlier this year. -

Meet the 2021 TMS Award Recipients
FEBRUARY 2021 jom.tms.org JAn officialO publication of The Minerals, Metals & Materials Society CELEBRATING EXCELLENCE: Meet the 2021 TMS Award Recipients Empowering Metallurgists, Process Engineers and Researchers Do you rely on handbook data? What if the materials data you need doesn’t exist? With Thermo-Calc you can: Calculate phase-based proper�es as a func�on of Base Decisions on scien�fically supported composi�on, temperature and �me models Fill in data gaps without resor�ng to costly, Accelerate materials development while �me-consuming experiments reducing risk Predict how actual vs nominal chemistries will affect Troubleshoot issues during materials processing property data Over 40 Thermodynamic and Kine�c Databases Choose from an extensive selec�on of thermodynamic and mobility databases in a range of materials, including: Steel and Fe-Alloys Nickel High Entropy Alloys 70 1000 Lath Total # of Alloys - 1032 1500 Plate RMS - 28.3 60 800 Epsilon 1450 Failure 50 1400 600 1350 40 400 1300 Calculated Ms [K]Calculated 30 200 Frequency 1250 20 Celsius Temperature, 1200 0 10 1150 1100 0 0 200 400 600 800 1000 1240 1245 1250 1255 1260 1265 1270 1275 1280 1285 0.00 0.05 0.10 0.15 0.20 0.25 0.30 Experimental Ms [K] Solidus temperature (°C) Mole Frac�on Al Comparison of calculated and experimental Varia�on in solidus temperature over 1000 Calculated phase diagram along the Ms temperatures for a wide range of steels composi�ons within alloy 718 specifica�on composi�on line of CoCrFeNi-Al Al Alloys Ti and TiAl Alloys Oxides 2.5 SiO2 Ti-6Al-4V [IMI] 1.0 [1961Wil] [1962McG] 0.9 2.0 Two Liq. -

Bull. Hist. Chem. 4
ll. t. Ch. 4 (8 2 Cpnd", . Krt., 2, 6, 848 (p.42 Grn. S l: K. THE HISTORY OF THE DEXTER AWARD jn, "r f lrt nd Mtl lrztn f In n th Mll f All lrd, SrO, nd O", Strtr nd ndn, rt I: h hrd d 6, , 880. K. r nd . , "ttnl Cntnt nd Eltr Aaron J. Ihde, University of Wisconsin pl Mnt f Sd lrd", Cnd. h., 6,4 (, 4646. h nnr f th rd, Mdt rllO (848, 6. ln, tprttn rnn nt t . E. ln, brn n Spn nd pld n prtnt rl n n Mrh 8. dtn n Spnh nvrt. At th l f th Spnh . K. jn, "frtn f In nd Mll d Upn Cvl Wr, h fld Spn nd trtd n rr n Mx, frttr t", . h, 28, 0, 6 nd . hr h flt br f th tnl lthn Eltrh., 28, 4, 0220. (th n Grn Inttt n Mx Ct. Althh h hd bn ntrtd n 8. A. E. vn Arl nd . d r, Chh ndn l htr f htr hl tll n Spn, tht ntrt flrd Elrrtth Erhnn, rzl, pz, . h txtb n Mx, hr h d xtnv td f th htr f br tn t jn pt n Erpn txt f tht d tllr n Clnl tn Ar. pblhd nr rfrn t h r tnbr th t n thr r. ppr n htr f hrntr nd f tllr nd . K. jn nd W. rnnbr, "Infln f Adrbd In th thr f vrl r n tnArn tllr. n th hthl Sntvn f Slvr rd", . -

RARE Velocimetry of Shear Banded Flow in Cylindrical Couette Geometry
RARE Velocimetry of Shear Banded Flow in Cylindrical Couette Geometry by Stefan Kuczera A thesis submitted to the Victoria University of Wellington in fulfilment of the requirements for the degree of Doctor of Philosophy Victoria University of Wellington 2015 Abstract A flow phenomena called ‘shear banding’ is often observed for a certain class of complex fluids, namely wormlike micellar solutions. Wormlike micelles are elongated flexible self-assembly struc- tures formed by the aggregation of amphiphiles, which may entangle into a dynamic network above a certain concentration threshold. The entanglement results in the sample having both solid-like (elastic) and liquid-like (viscous) properties, an ambiguity commonly found in com- plex fluids. Under certain shear conditions, the flow couples with the structure of the micellar network, leading to the formation of (shear) bands with differing viscosity. The principle goal of this work is to address open questions regarding the temporal and spatial stability of shear banded flow. Shear banding is often studied in cylindrical Couette cells, where the fluid is sheared in a gap between differentially rotating concentric cylinders. For the sake of an accurate description of the flow in such a shear cell, the methodology for a 2D Nuclear Magnetic Resonance (NMR) velocimetry technique (known as PGSE-RARE), which offers high temporal and spatial resolution, is improved and refined. Two main challenges are identified and overcome. The first concerns the fact that the velocity imaging process operates on a Cartesian grid, whereas the flow in the Couette cell is of cylindrical symmetry. Numerical calculations and NMR simulations based on the Bloch equations, as well as experimental evidence, give insight on the appropriate selection of the fluid volume over which velocity information is accumulated and the preferred scheme through which the NMR image is acquired in the so-called k-space. -

Sustainable Economic Growth for New Zealand: an Optimistic Myth-Busting Perspective
contributingpaper 1 Sustainable economic growth for New Zealand: An optimistic myth-busting perspective Sir Paul Callaghan 31 March 2011 This paper was prepared by Sir Paul Callaghan for the participants of the StrategyNZ: Mapping our Future workshop held in March 2011. Long-term vision is something we tend to avoid in New Zealand, with the possible exception of Måori, who have greater reason to focus on the development of their assets for future generations of mokopuna. But I will argue here that vision is essential to any strategy aimed at enhancing prosperity. It is my belief that we are poor because we choose to be poor, and that what holds us back are self-serving but dishonest myths. The first myth is that we are an egalitarian society, a great place to bring up children. But in income disparity, child mortality, imprisonment rates and most other negative social indicators, we are among the worst in the OECD. The second myth is that we are clean and green. In truth, the reality is altogether different. Like other developed countries we have despoiled our environment to eke out a measure of prosperity, and we therefore have no moral high ground from which to preach to others. Our valuable dairy industry severely impacts our rivers and lakes. Our pastoral industries are significant emitters of greenhouse gases. The third myth is that we, as New Zealanders, do not need prosperity, that we have ‘lifestyle’ instead. But we complain that our health system cannot afford to meet our needs and that our infrastructure is decrepit. -

Chemistry in New Zealand April 2007 New Zealand Institute of Chemistry Supporting Chemical Sciences April News NZIC News NEW ZEALAND INSTITUTE of CHEMISTRY
Inside Volume 71, No.1, April 2007 Articles and Features 2 NZIC April News 5 IC07 - Hobart 6 Molecular Materials Research within the MacDiarmid Institute Keith C. Gordon 9 Soft Matter in the MacDiarmid Institute Kathryn M. McGrath 14 Recent Chemistry of Advanced Inorganic and Hybrid Materials at the MacDiarmid Institute Kenneth J. D. MacKenzie 19 The Chemical History of Anaesthesia Joanna Wojnar 26 Obituary – Denis James Hogan 27 Denis Hogan on Chemical Education – The Last Comments 29 NZIC Conference – Rotorua Regular Columns 24 Conference Calendar 25 Patent Proze 32 New Zealand Science Scene 32 Chemistry Behind the News Advertisers Index Inside Front Cover Biolab Back Cover Phenomenex Inside Back Cover ChemEd007 5 IC07 Conference 1 Chemistry in New Zealand April 2007 New Zealand Institute of Chemistry supporting chemical sciences April News NZIC News NEW ZEALAND INSTITUTE OF CHEMISTRY 75th Anniversary – NZIC is 75 in 2007 NEWS cially shellfish toxins. This outstand- regard with which both Murray and Members will be aware of the death ing achievement, the first to a New John are held by their international of Victoria alumnus and Nobel Lau- Zealander, recognises an outstanding community. reate, Professor Alan MacDiarmid on synthetic chemist at the top of her field. The annual P B D De La Mare Me- February 7 from the numerous media morial Lecture on constructing reports (see earlier in this issue also). Dr Sheila Woodgate received a rich- quaternary carbon stereocenters: As announced in the December is- ly-deserved University Innovation in methods development and natural sue, the 2007 75th Jubilee President Teaching Award in recognition of her products total synthesis was given in is Jan Wikaira of the University of development of Best Choice. -

2010 Academy Annual Report
This document has been created from historical website content. 2010 Academy Annual Report The 2010 Academy Annual Report details the accomplishments of the Academy of the Royal Society of New Zealand throughout the year. Chair’s foreword In January 2010 the final act of “embracing the Humanities” within the Royal Society took place with the signing of an MOU between Te Whainga Aronui The Council for the Humanities and Te Apārangi the Royal Society of New Zealand. President Dr Garth Carnaby described this as landmark decision for both organisations. “For the first time there will be an organisation in New Zealand that promotes excellence in research and scholarship across all the disciplines and areas of knowledge.” The CV’s of the existing fellows of the Council for the Humanities were reviewed by the Academy Executive and these people were admitted to the Fellowship by a special resolution at the Fellow’s AGM. The Academy has continued to provide “information pieces” and forward- looking contributions on present and emerging debates that were introduced in 2009. In 2010, 2 information statements were prepared. Sea Level Rise: Emerging Issues recounted the significant changes in the scientific understanding of this issue since the 2007 Assessment Report by the IPCC. The Darfield Earthquake: The Value of Long-term Research was produced to document the research conducted in New Zealand over many years on how to construct and strengthen buildings to improve performance during large earthquakes. The application of this science and engineering undoubtedly played a big role in limiting the damage and injury caused by the 2010 Christchurch earthquake. -
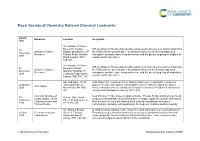
RSC Branding
Royal Society of Chemistry National Chemical Landmarks Award Honouree Location Inscription Date The Institute of Cancer Research, Chester ICR scientists on this site and elsewhere pioneered numerous new cancer drugs from 10 Institute of Cancer Beatty Laboratories, 237 the 1950s until the present day – including the discovery of chemotherapy drug December Research Fulham Road, Chelsea carboplatin, prostate cancer drug abiraterone and the genetic targeting of olaparib for 2018 Road, London, SW3 ovarian and breast cancer. 6JB, UK The Institute of Cancer ICR scientists on this site and elsewhere pioneered numerous new cancer drugs from 10 Research, Royal Institute of Cancer the 1950s until the present day – including the discovery of chemotherapy drug December Marsden Hospital, 15 Research carboplatin, prostate cancer drug abiraterone and the genetic targeting of olaparib for 2018 Cotswold Road, Sutton, ovarian and breast cancer. London, SM2 5NG, UK Ape and Apple, 28-30 John Dalton Street was opened in 1846 by Manchester Corporation in honour of 26 October John Dalton Street, famous chemist, John Dalton, who in Manchester in 1803 developed the Atomic John Dalton 2016 Manchester, M2 6HQ, Theory which became the foundation of modern chemistry. President of Manchester UK Literary and Philosophical Society 1816-1844. Chemical structure of Near this site in 1903, James Colquhoun Irvine, Thomas Purdie and their team found 30 College Gate, North simple sugars, James a way to understand the chemical structure of simple sugars like glucose and lactose. September Street, St Andrews, Fife, Colquhoun Irvine and Over the next 18 years this allowed them to lay the foundations of modern 2016 KY16 9AJ, UK Thomas Purdie carbohydrate chemistry, with implications for medicine, nutrition and biochemistry. -

Robert Burns Woodward
The Life and Achievements of Robert Burns Woodward Long Literature Seminar July 13, 2009 Erika A. Crane “The structure known, but not yet accessible by synthesis, is to the chemist what the unclimbed mountain, the uncharted sea, the untilled field, the unreached planet, are to other men. The achievement of the objective in itself cannot but thrill all chemists, who even before they know the details of the journey can apprehend from their own experience the joys and elations, the disappointments and false hopes, the obstacles overcome, the frustrations subdued, which they experienced who traversed a road to the goal. The unique challenge which chemical synthesis provides for the creative imagination and the skilled hand ensures that it will endure as long as men write books, paint pictures, and fashion things which are beautiful, or practical, or both.” “Art and Science in the Synthesis of Organic Compounds: Retrospect and Prospect,” in Pointers and Pathways in Research (Bombay:CIBA of India, 1963). Robert Burns Woodward • Graduated from MIT with his Ph.D. in chemistry at the age of 20 Woodward taught by example and captivated • A tenured professor at Harvard by the age of 29 the young... “Woodward largely taught principles and values. He showed us by • Published 196 papers before his death at age example and precept that if anything is worth 62 doing, it should be done intelligently, intensely • Received 24 honorary degrees and passionately.” • Received 26 medals & awards including the -Daniel Kemp National Medal of Science in 1964, the Nobel Prize in 1965, and he was one of the first recipients of the Arthur C. -

St Catherine's College Oxford
MESSAGES The Year St Catherine’s College . Oxford 2013 ST CATHERINE’S COLLEGE 2013/75 MESSAGES Master and Fellows 2013 MASTER Susan C Cooper, MA (BA Richard M Berry, MA, DPhil Bart B van Es (BA, MPhil, Angela B Brueggemann, Gordon Gancz, BM BCh, MA Collby Maine, PhD California) Tutor in Physics PhD Camb) DPhil (BSc St Olaf, MSc Fellow by Special Election Professor Roger W Professor of Experimental Reader in Condensed Tutor in English Iowa) College Doctor Ainsworth, MA, DPhil, Physics Matter Physics Senior Tutor Fellow by Special Election in FRAeS Biological Sciences Geneviève A D M Peter R Franklin, MA (BA, Ashok I Handa, MA (MB BS Tommaso Pizzari, MA (BSc Wellcome Trust Career Helleringer (Maîtrise FELLOWS DPhil York) Lond), FRCS Aberd, PhD Shef) Development Fellow ESSEC, JD Columbia, Maîtrise Tutor in Music Fellow by Special Election in Tutor in Zoology Sciences Po, Maîtrise, Richard J Parish, MA, DPhil Professor of Music Medicine James E Thomson, MChem, Doctorat Paris-I Panthéon- (BA Newc) Reader in Surgery Byron W Byrne, MA, DPhil DPhil Sorbonne, Maîtrise Paris-II Tutor in French John Charles Smith, MA Tutor for Graduates (BCom, BEng Western Fellow by Special Election in Panthéon-Assas) Philip Spencer Fellow Tutor in French Linguistics Australia) Chemistry Fellow by Special Election Professor of French President of the Senior James L Bennett, MA (BA Tutor in Engineering Science in Law (Leave H14) Common Room Reading) Tutor for Admissions Andrew J Bunker, MA, DPhil Leverhulme Trust Early Fellow by Special Election Tutor in Physics -

Sir John Warcup Cornforth
Sir John Warcup Cornforth The degree of Doctor of Science (honoris causa) was conferred upon Sir John Warcup Cornforth at a ceremony held in the Great Hall on 2 November 1977. Sir John Warcup Cornforth, photo G3_224_1122, University of Sydney Archives. Citation A Nobel Prize-winning chemist who has been totally deaf for the past 40 years will be awarded the honorary degree of Doctor of Science by the University at a cemony in the Great Hall next Wednesday, November 2, 1977. He is Sir John Cornforth, 60, who was awarded the Nobel Prize for Chemistry in 1975 for his work on the stereochemistry of enzyme-catalysed reactions. He is currently the Royal Society Research Professor at the University of Sussex, and during his four-week visit to Australia he will deliver lectures in Sydney, Canberra, Melbourne and Adelaide. Sir John entered this University at the age of 16. The first signs of his deafness (from otosclerosis) had begun when he was 10, and although he had been able to profit from teaching at Sydney Boys' School, he was unable to hear any lecture at the University. He was, however, attracted by laboratory work in organic chemistry (which he had done in an improvised laboratory at home since the age of 14) and by the availability of original chemical literature. In 1937 he graduated with first-class honours and the University medal. After a year of postgraduate research he won a scholarship to work at Oxford. Two such scholarships were awarded each year, and the other was won by Rita Harradence, also of Sydney and also a winner of the University medal. -

Robert Burns Woodward 1917–1979
NATIONAL ACADEMY OF SCIENCES ROBERT BURNS WOODWARD 1917–1979 A Biographical Memoir by ELKAN BLOUT Any opinions expressed in this memoir are those of the author and do not necessarily reflect the views of the National Academy of Sciences. Biographical Memoirs, VOLUME 80 PUBLISHED 2001 BY THE NATIONAL ACADEMY PRESS WASHINGTON, D.C. ROBERT BURNS WOODWARD April 10, 1917–July 8, 1979 BY ELKAN BLOUT OBERT BURNS WOODWARD was the preeminent organic chemist Rof the twentieth century. This opinion is shared by his colleagues, students, and by other distinguished chemists. Bob Woodward was born in Boston, Massachusetts, and was an only child. His father died when Bob was less than two years old, and his mother had to work hard to support her son. His early education was in the Quincy, Massachusetts, public schools. During this period he was allowed to skip three years, thus enabling him to finish grammar and high schools in nine years. In 1933 at the age of 16, Bob Woodward enrolled in the Massachusetts Institute of Technology to study chemistry, although he also had interests at that time in mathematics, literature, and architecture. His unusual talents were soon apparent to the MIT faculty, and his needs for individual study and intensive effort were met and encouraged. Bob did not disappoint his MIT teachers. He received his B.S. degree in 1936 and completed his doctorate in the spring of 1937, at which time he was only 20 years of age. Immediately following his graduation Bob taught summer school at the University of Illinois, but then returned to Harvard’s Department of Chemistry to start a productive period with an assistantship under Professor E.