Sir James Fraser Stoddart Baran Lab GM 2010-08-14
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Geoffrey Wilkinson
THE LONG SEARCH FOR STABLE TRANSITION METAL ALKYLS Nobel Lecture, December 11, 1973 by G EOFFREY W ILKINSON Imperial College of Science & Technology, London, England Chemical compounds in which there is a single bond between a saturated car- bon atom and a transition metal atom are of unusual importance. Quite aside from the significance and role in Nature of the cobalt to carbon bonds in the vitamin B 12 system and possible metal to carbon bonds in other biological systems, we need only consider that during the time taken to deliver this lec- ture, many thousands, if not tens of thousands of tons of chemical compounds are being transformed or synthesised industrially in processes which at some stage involve a transition metal to carbon bond. The nonchemist will pro- bably be most familiar with polyethylene or polypropylene in the form of do- mestic utensils, packaging materials, children’s toys and so on. These materials are made by Ziegler-Natta* or Philipps’ catalysis using titanium and chro- mium respectively. However, transition metal compounds are used as catalysts in the synthesis of synthetic rubbers and other polymers, and of a variety of simple compounds used as industrial solvents or intermediates. For example alcohols are made from olefins, carbon monoxide and hydrogen by use of cobalt or rhodium catalysts, acetic acid is made by carbonylation of methanol using rhodium catalysts and acrylonitrile is dimerised to adiponitrile (for nylon) by nickel catalysts. We should also not forget that the huge quantities of petroleum hydrocarbons processed by the oil and petrochemical industry are re-formed over platinum, platinum-rhenium or platinum-germanium sup- ported on alumina. -

Historical Group
Historical Group NEWSLETTER and SUMMARY OF PAPERS No. 64 Summer 2013 Registered Charity No. 207890 COMMITTEE Chairman: Prof A T Dronsfield | Prof J Betteridge (Twickenham, 4, Harpole Close, Swanwick, Derbyshire, | Middlesex) DE55 1EW | Dr N G Coley (Open University) [e-mail [email protected]] | Dr C J Cooksey (Watford, Secretary: Prof. J. W. Nicholson | Hertfordshire) School of Sport, Health and Applied Science, | Prof E Homburg (University of St Mary's University College, Waldegrave | Maastricht) Road, Twickenham, Middlesex, TW1 4SX | Prof F James (Royal Institution) [e-mail: [email protected]] | Dr D Leaback (Biolink Technology) Membership Prof W P Griffith | Dr P J T Morris (Science Museum) Secretary: Department of Chemistry, Imperial College, | Mr P N Reed (Steensbridge, South Kensington, London, SW7 2AZ | Herefordshire) [e-mail [email protected]] | Dr V Quirke (Oxford Brookes Treasurer: Dr J A Hudson | University) Graythwaite, Loweswater, Cockermouth, | Prof. H. Rzepa (Imperial College) Cumbria, CA13 0SU | Dr. A Sella (University College) [e-mail [email protected]] Newsletter Dr A Simmons Editor Epsom Lodge, La Grande Route de St Jean, St John, Jersey, JE3 4FL [e-mail [email protected]] Newsletter Dr G P Moss Production: School of Biological and Chemical Sciences, Queen Mary University of London, Mile End Road, London E1 4NS [e-mail [email protected]] http://www.chem.qmul.ac.uk/rschg/ http://www.rsc.org/membership/networking/interestgroups/historical/index.asp 1 RSC Historical Group Newsletter No. 64 Summer 2013 Contents From the Editor 2 Obituaries 3 Professor Colin Russell (1928-2013) Peter J.T. -
RSC Branding
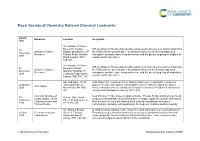
Royal Society of Chemistry National Chemical Landmarks Award Honouree Location Inscription Date The Institute of Cancer Research, Chester ICR scientists on this site and elsewhere pioneered numerous new cancer drugs from 10 Institute of Cancer Beatty Laboratories, 237 the 1950s until the present day – including the discovery of chemotherapy drug December Research Fulham Road, Chelsea carboplatin, prostate cancer drug abiraterone and the genetic targeting of olaparib for 2018 Road, London, SW3 ovarian and breast cancer. 6JB, UK The Institute of Cancer ICR scientists on this site and elsewhere pioneered numerous new cancer drugs from 10 Research, Royal Institute of Cancer the 1950s until the present day – including the discovery of chemotherapy drug December Marsden Hospital, 15 Research carboplatin, prostate cancer drug abiraterone and the genetic targeting of olaparib for 2018 Cotswold Road, Sutton, ovarian and breast cancer. London, SM2 5NG, UK Ape and Apple, 28-30 John Dalton Street was opened in 1846 by Manchester Corporation in honour of 26 October John Dalton Street, famous chemist, John Dalton, who in Manchester in 1803 developed the Atomic John Dalton 2016 Manchester, M2 6HQ, Theory which became the foundation of modern chemistry. President of Manchester UK Literary and Philosophical Society 1816-1844. Chemical structure of Near this site in 1903, James Colquhoun Irvine, Thomas Purdie and their team found 30 College Gate, North simple sugars, James a way to understand the chemical structure of simple sugars like glucose and lactose. September Street, St Andrews, Fife, Colquhoun Irvine and Over the next 18 years this allowed them to lay the foundations of modern 2016 KY16 9AJ, UK Thomas Purdie carbohydrate chemistry, with implications for medicine, nutrition and biochemistry. -

Robert Burns Woodward
The Life and Achievements of Robert Burns Woodward Long Literature Seminar July 13, 2009 Erika A. Crane “The structure known, but not yet accessible by synthesis, is to the chemist what the unclimbed mountain, the uncharted sea, the untilled field, the unreached planet, are to other men. The achievement of the objective in itself cannot but thrill all chemists, who even before they know the details of the journey can apprehend from their own experience the joys and elations, the disappointments and false hopes, the obstacles overcome, the frustrations subdued, which they experienced who traversed a road to the goal. The unique challenge which chemical synthesis provides for the creative imagination and the skilled hand ensures that it will endure as long as men write books, paint pictures, and fashion things which are beautiful, or practical, or both.” “Art and Science in the Synthesis of Organic Compounds: Retrospect and Prospect,” in Pointers and Pathways in Research (Bombay:CIBA of India, 1963). Robert Burns Woodward • Graduated from MIT with his Ph.D. in chemistry at the age of 20 Woodward taught by example and captivated • A tenured professor at Harvard by the age of 29 the young... “Woodward largely taught principles and values. He showed us by • Published 196 papers before his death at age example and precept that if anything is worth 62 doing, it should be done intelligently, intensely • Received 24 honorary degrees and passionately.” • Received 26 medals & awards including the -Daniel Kemp National Medal of Science in 1964, the Nobel Prize in 1965, and he was one of the first recipients of the Arthur C. -

St Catherine's College Oxford
MESSAGES The Year St Catherine’s College . Oxford 2013 ST CATHERINE’S COLLEGE 2013/75 MESSAGES Master and Fellows 2013 MASTER Susan C Cooper, MA (BA Richard M Berry, MA, DPhil Bart B van Es (BA, MPhil, Angela B Brueggemann, Gordon Gancz, BM BCh, MA Collby Maine, PhD California) Tutor in Physics PhD Camb) DPhil (BSc St Olaf, MSc Fellow by Special Election Professor Roger W Professor of Experimental Reader in Condensed Tutor in English Iowa) College Doctor Ainsworth, MA, DPhil, Physics Matter Physics Senior Tutor Fellow by Special Election in FRAeS Biological Sciences Geneviève A D M Peter R Franklin, MA (BA, Ashok I Handa, MA (MB BS Tommaso Pizzari, MA (BSc Wellcome Trust Career Helleringer (Maîtrise FELLOWS DPhil York) Lond), FRCS Aberd, PhD Shef) Development Fellow ESSEC, JD Columbia, Maîtrise Tutor in Music Fellow by Special Election in Tutor in Zoology Sciences Po, Maîtrise, Richard J Parish, MA, DPhil Professor of Music Medicine James E Thomson, MChem, Doctorat Paris-I Panthéon- (BA Newc) Reader in Surgery Byron W Byrne, MA, DPhil DPhil Sorbonne, Maîtrise Paris-II Tutor in French John Charles Smith, MA Tutor for Graduates (BCom, BEng Western Fellow by Special Election in Panthéon-Assas) Philip Spencer Fellow Tutor in French Linguistics Australia) Chemistry Fellow by Special Election Professor of French President of the Senior James L Bennett, MA (BA Tutor in Engineering Science in Law (Leave H14) Common Room Reading) Tutor for Admissions Andrew J Bunker, MA, DPhil Leverhulme Trust Early Fellow by Special Election Tutor in Physics -

Los Premios Nobel De Química
Los premios Nobel de Química MATERIAL RECOPILADO POR: DULCE MARÍA DE ANDRÉS CABRERIZO Los premios Nobel de Química El campo de la Química que más premios ha recibido es el de la Quí- mica Orgánica. Frederick Sanger es el único laurea- do que ganó el premio en dos oca- siones, en 1958 y 1980. Otros dos también ganaron premios Nobel en otros campos: Marie Curie (física en El Premio Nobel de Química es entregado anual- 1903, química en 1911) y Linus Carl mente por la Academia Sueca a científicos que so- bresalen por sus contribuciones en el campo de la Pauling (química en 1954, paz en Física. 1962). Seis mujeres han ganado el Es uno de los cinco premios Nobel establecidos en premio: Marie Curie, Irène Joliot- el testamento de Alfred Nobel, en 1895, y que son dados a todos aquellos individuos que realizan Curie (1935), Dorothy Crowfoot Ho- contribuciones notables en la Química, la Física, la dgkin (1964), Ada Yonath (2009) y Literatura, la Paz y la Fisiología o Medicina. Emmanuelle Charpentier y Jennifer Según el testamento de Nobel, este reconocimien- to es administrado directamente por la Fundación Doudna (2020) Nobel y concedido por un comité conformado por Ha habido ocho años en los que no cinco miembros que son elegidos por la Real Aca- demia Sueca de las Ciencias. se entregó el premio Nobel de Quí- El primer Premio Nobel de Química fue otorgado mica, en algunas ocasiones por de- en 1901 al holandés Jacobus Henricus van't Hoff. clararse desierto y en otras por la Cada destinatario recibe una medalla, un diploma y situación de guerra mundial y el exi- un premio económico que ha variado a lo largo de los años. -

Weak Functional Group Interactions Revealed Through Metal-Free Active Template Rotaxane Synthesis
ARTICLE https://doi.org/10.1038/s41467-020-14576-7 OPEN Weak functional group interactions revealed through metal-free active template rotaxane synthesis Chong Tian 1,2, Stephen D.P. Fielden 1,2, George F.S. Whitehead 1, Iñigo J. Vitorica-Yrezabal1 & David A. Leigh 1* 1234567890():,; Modest functional group interactions can play important roles in molecular recognition, catalysis and self-assembly. However, weakly associated binding motifs are often difficult to characterize. Here, we report on the metal-free active template synthesis of [2]rotaxanes in one step, up to 95% yield and >100:1 rotaxane:axle selectivity, from primary amines, crown ethers and a range of C=O, C=S, S(=O)2 and P=O electrophiles. In addition to being a simple and effective route to a broad range of rotaxanes, the strategy enables 1:1 interactions of crown ethers with various functional groups to be characterized in solution and the solid state, several of which are too weak — or are disfavored compared to other binding modes — to be observed in typical host–guest complexes. The approach may be broadly applicable to the kinetic stabilization and characterization of other weak functional group interactions. 1 Department of Chemistry, University of Manchester, Manchester M13 9PL, UK. 2These authors contributed equally: Chong Tian, Stephen D. P. Fielden. *email: [email protected] NATURE COMMUNICATIONS | (2020) 11:744 | https://doi.org/10.1038/s41467-020-14576-7 | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-020-14576-7 he bulky axle end-groups of rotaxanes mechanically lock To explore the scope of this unexpected method of rotaxane Trings onto threads, preventing the dissociation of the synthesis, here we carry out a study of the reaction with a series of components even if the interactions between them are not related electrophiles. -

Cv Mlhg 2015
CURRICULUM VITAE Name: GREEN, Malcolm Leslie Hodder Address: St Catherine's College, Oxford or Inorganic Chemistry Laboratory South Parks Road OXFORD, OX1 3QR Date of Birth: 16/4/36, Eastleigh, Hampshire Nationality: British Marital Status: Married. Three children Degrees: B.Sc.(Hons), London; D.I.C., M.A.(Cantab), M.A.(Oxon), C.Chem., F.R.S.C., Ph.D., F.R.S. ACADEMIC CAREER 1953-56 Acton Technical College, University of London, B.Sc Hons. Chemistry 1956-59 Imperial College of Science and Technology, London; D.I.C. Ph.D. in chemistry. Supervisor Professor Sir G. Wilkinson 1959-60 Post-doctoral Research Associates Fellow. Imperial College of Science and Technology 1960-63 Assistant Lecturer in Inorganic Chemistry at Cambridge University 1961 Fellow of Corpus Christi College, Cambridge 1963 Sepcentenary Fellow of Inorganic Chemistry, Balliol College, Oxford and Departmental Demonstrator, University of Oxford 1965 University Lecturer, University of Oxford 1971 Visiting Professor, University of Western Ontario (Spring Term) 1972 Visiting Professor, Ecole de Chimie and Institute des Substances Naturelles, Paris (six months) 1973 A.P. Sloan Visiting Professor, Harvard University, (Spring Semester) 1979-84 Appointed to the British Gas Royal Society Senior Research Fellowship 1981 Sherman Fairchild Visiting Scholar at the California Institute of Technology(4 months) 1984 Re-appointed British Gas Royal Society Senior Research Fellow (1984-6) 1987 Vice-master, Balliol College, Oxford (T.T.) 1989 Appointed Professor of Inorganic Chemistry and Head of Department, Oxford University Fellow of St Catherine's College, Oxford 2004- present Emeritus Research Professor in the Inorganic Chemistry Laboratory, Oxford University Emeritus Fellow of Balliol College and St Catherine’s College Publications Two text books, 646 refereed papers and 8 patents. -

Guidelines and Suggested Title List for Undergraduate Chemistry Libraries, Serial Publication Number 44
DOCUMENT RESUME ED 040 037 SE 008 009 AUTHOR Marquardt, D. N., Ed. TITLE Guidelines and Suggested Title List for Undergraduate Chemistry Libraries, Serial Publication Number 44. INSTITUTION Advisory Council on Coll, Chemistry. PUB DATE Sep 69 NOTE 44p. AVAILABLE FROM Advisory Council on College Chemistry, Dept. of Chemistry, Stanford Univ., Stanford,California 94305 (free) EDRS PRICE EDRS P-: ice MF.40.25 HC-$2.30 DESCRIPTORS Advisory Committees, *Bibliographies,Booklists, *Chemistry, *College Science, *LibraryGuides, Research Reviews (Publications): *Resource Materials, Scholarly Journals IDENTIFIERS Advisory Council on College Chemistry ABSTRACT Contained are guidelines and an extensivelist of books and journals suitable for anundergraduate chemistry library. The guidelines are concerned with theorganization and acquisition policy of chemistry libraries, and withinter-library loan and photoduplication services. Various sections of the reportdeal with journals and abstracts, review serials,foreign language titles, U.S. Government publications and a suggestedtitles list. The books in the titles list are in the areas of analytical,biological, inorganic, organic and physical chemistry. Ingeneral, introductory texts have not been included. The list isarranged alphabetically with entries by author or editor unless the workis better known by title. The library of Congress classification numberand the Dewey Decimal classification number, when available, aregiven for each entry. Book prices are also given. The reportconcludes with a directory of publishers and dealers. This report shouldbe most useful for college libraries, science teachers, and students. (LC) 0 GUIDELINES AND SUGGESTEDTITLE LIST for t...UNDERGRADUATE CHEMISTRY LIBRARIES M CI Revised 1969 Co Co A Report Authorized by the ADVISORY COUNCIL ON COLLEGE CHEMISTRY Edited by D. -

Sir John Warcup Cornforth
Sir John Warcup Cornforth The degree of Doctor of Science (honoris causa) was conferred upon Sir John Warcup Cornforth at a ceremony held in the Great Hall on 2 November 1977. Sir John Warcup Cornforth, photo G3_224_1122, University of Sydney Archives. Citation A Nobel Prize-winning chemist who has been totally deaf for the past 40 years will be awarded the honorary degree of Doctor of Science by the University at a cemony in the Great Hall next Wednesday, November 2, 1977. He is Sir John Cornforth, 60, who was awarded the Nobel Prize for Chemistry in 1975 for his work on the stereochemistry of enzyme-catalysed reactions. He is currently the Royal Society Research Professor at the University of Sussex, and during his four-week visit to Australia he will deliver lectures in Sydney, Canberra, Melbourne and Adelaide. Sir John entered this University at the age of 16. The first signs of his deafness (from otosclerosis) had begun when he was 10, and although he had been able to profit from teaching at Sydney Boys' School, he was unable to hear any lecture at the University. He was, however, attracted by laboratory work in organic chemistry (which he had done in an improvised laboratory at home since the age of 14) and by the availability of original chemical literature. In 1937 he graduated with first-class honours and the University medal. After a year of postgraduate research he won a scholarship to work at Oxford. Two such scholarships were awarded each year, and the other was won by Rita Harradence, also of Sydney and also a winner of the University medal. -

Robert Burns Woodward 1917–1979
NATIONAL ACADEMY OF SCIENCES ROBERT BURNS WOODWARD 1917–1979 A Biographical Memoir by ELKAN BLOUT Any opinions expressed in this memoir are those of the author and do not necessarily reflect the views of the National Academy of Sciences. Biographical Memoirs, VOLUME 80 PUBLISHED 2001 BY THE NATIONAL ACADEMY PRESS WASHINGTON, D.C. ROBERT BURNS WOODWARD April 10, 1917–July 8, 1979 BY ELKAN BLOUT OBERT BURNS WOODWARD was the preeminent organic chemist Rof the twentieth century. This opinion is shared by his colleagues, students, and by other distinguished chemists. Bob Woodward was born in Boston, Massachusetts, and was an only child. His father died when Bob was less than two years old, and his mother had to work hard to support her son. His early education was in the Quincy, Massachusetts, public schools. During this period he was allowed to skip three years, thus enabling him to finish grammar and high schools in nine years. In 1933 at the age of 16, Bob Woodward enrolled in the Massachusetts Institute of Technology to study chemistry, although he also had interests at that time in mathematics, literature, and architecture. His unusual talents were soon apparent to the MIT faculty, and his needs for individual study and intensive effort were met and encouraged. Bob did not disappoint his MIT teachers. He received his B.S. degree in 1936 and completed his doctorate in the spring of 1937, at which time he was only 20 years of age. Immediately following his graduation Bob taught summer school at the University of Illinois, but then returned to Harvard’s Department of Chemistry to start a productive period with an assistantship under Professor E. -
Derek HR Barton
news and views Obituary reactions on hydrocarbons. Derek H. R. Barton (1918–98) Once again in 1985 the black hole of retirement loomed, and once again Barton Polymath of organic was not prepared to retire. This brought him to Texas A&M. Why? Quite simply, chemistry because we offered him what he wanted: TEXAS A&M With the death of Sir Derek Barton, on 16 not a name chair, not a big office, not the March, we will miss not only a great role of being a brilliant but non-functional chemical intellect but a fascinating and adornment. We offered a regular full 8 delightful human being. He was always professorship, a decent office and all the full of plans and receptive to ideas, and he lab space he needed — which turned out to took pleasure in the company of his be a lot. Barton was thus able to continue colleagues. Like his long-time colleague his unflagging study of Gif chemistry and Geoffrey Wilkinson, he passed away the invention of new reactions. suddenly and unexpectedly of a heart His time in College Station was both attack. In both cases this was the best way, sad and happy. Not long after his arrival as neither would have tolerated a state of Christiane developed cancer, and after forced inactivity. several very painful years for both, she Derek Barton was born in Gravesend, died. Barton threw himself more than ever England, on 8 September 1918. His family into his work, but he soon had the great were in what he referred to as “the wood good fortune to remarry (to Judith Cobb, a business”.