National Sample Transportation System (NSTS)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

11010329.Pdf
THE RISE, CONSOLIDATION AND DISINTEGRATION OF DLAMINI POWER IN SWAZILAND BETWEEN 1820 AND 1889. A study in the relationship of foreign affairs to internal political development. Philip Lewis Bonner. ProQuest Number: 11010329 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 11010329 Published by ProQuest LLC(2018). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 ABSTRACT The Swazi kingdom grew out of the pressures associated with competition for trade and for the rich resources of Shiselweni. While centred on this area it acquired some of its characteristic features - notably a regimental system, and the dominance of a Dlamini aristocracy. Around 1815 the Swazi came under pressure from the South, and were forced to colonise the land lying north of the Lusutfu. Here they remained for some years a nation under arms, as they plundered local peoples, and were themselves swept about by the currents of the Mfecane. In time a more settled administration emerged, as the aristocracy spread out from the royal centres at Ezulwini, and this process accelerated under Mswati as he subdued recalcitrant chiefdoms, and restructured the regiments. -

Lubombo Health Performance Report 2015
SI A Y I N Q A B KINGDOM OF SWAZILAND MINISTRY OF HEALTH REGIONAL HEALTH PERFORMANCE REPORT LUBOMBO REGION 2015 Strategic Information Department This publication was produced with the support of the United States Agency for International Development (USAID) under the terms of MEASURE Evaluation cooperative agreement AID-0AA-L-14-00004. Views expressed are not necessarily those of USAID or the United States government TABLE OF CONTENTS List of acronyms...........................................................................................................................................v Acknowledgements.....................................................................................................................................vi Executive summary....................................................................................................................................vii Annual Regional Objectives......................................................................................................................viii CHAPTER 1: Introduction...............................................................................................................1 1.1 Regional background.....................................................................................................2 1.1.1 Geographic Location......................................................................................................2 1.1.2 Population profile...........................................................................................................2 -

Page 1 2018 NATIONAL ELECTIONS
2018 NATIONAL ELECTIONS - POLLING STATIONS REGION INKHUNDLA POLLING DIVISION HHOHHO HHUKWINI Dlangeni HHUKWINI KaSiko HHUKWINI Lamgabhi HHUKWINI Lamgabhi HHUKWINI Sitseni LOBAMBA Elangeni LOBAMBA Ezulwini LOBAMBA Ezulwini LOBAMBA Ezulwini LOBAMBA Lobamba LOBAMBA Nkhanini LOBAMBA Nkhanini LOBAMBA Zabeni LOBAMBA Zabeni MADLANGEMPISI Dvokolwako / Ekuphakameni MADLANGEMPISI Dvokolwako / Ekuphakameni MADLANGEMPISI Ekukhulumeni/ Mandlangempisi MADLANGEMPISI Ekukhulumeni/ Mandlangempisi MADLANGEMPISI Gucuka MADLANGEMPISI Mavula MADLANGEMPISI Nyonyane/ Maguga MADLANGEMPISI Tfuntini/Buhlebuyeza MADLANGEMPISI Tfuntini/Buhlebuyeza MADLANGEMPISI Tfuntini/Buhlebuyeza MADLANGEMPISI Tfuntini/Buhlebuyeza MADLANGEMPISI Zandondo MADLANGEMPISI Zandondo MAPHALALENI Dlozini MAPHALALENI Madlolo MAPHALALENI Maphalaleni MAPHALALENI Mcengeni MAPHALALENI Mfeni MAPHALALENI Nsingweni MAPHALALENI Nsingweni MAYIWANE Herefords MAYIWANE Mavula MAYIWANE Mfasini MAYIWANE Mkhuzweni MAYIWANE Mkhuzweni MAYIWANE Mkhweni MBABANE EAST Fontein MBABANE EAST Fontein MBABANE EAST Mdzimba/Lofokati MBABANE EAST Mdzimba/Lofokati MBABANE EAST Msunduza MBABANE EAST Msunduza MBABANE EAST Msunduza MBABANE EAST Sidwashini MBABANE EAST Sidwashini MBABANE EAST Sidwashini MBABANE EAST Sidwashini MBABANE WEST Mangwaneni MBABANE WEST Mangwaneni MBABANE WEST Mangwaneni MBABANE WEST Manzana MBABANE WEST Nkwalini MBABANE WEST Nkwalini MBABANE WEST Nkwalini MBABANE WEST Nkwalini MHLANGATANE Emalibeni MHLANGATANE Mangweni MHLANGATANE Mphofu MHLANGATANE Mphofu MHLANGATANE Ndvwabangeni MHLANGATANE -

THE STATE of WASH FINANCING in EASTERN and SOUTHERN AFRICA Eswatini Country Level Assessment
eSwatini THE STATE OF WASH FINANCING IN EASTERN AND SOUTHERN AFRICA Eswatini Country Level Assessment 1 Authors: Oliver Jones, Oxford Policy Management, in collaboration with Agua Consult and Blue Chain Consulting, Oxford, UK. Reviewers: Samuel Godfrey and Bernard Keraita, UNICEF Regional Office for Eastern and Southern Africa, Nairobi, Kenya and Boniswa Dladla (UNICEF Eswatini Country Office). Acknowledgement The author wishes to thank all other contributors from the UNICEF Eswatini Country Office, Government of the Kingdom of Eswatini and development partners. Special thanks go to UNICEF Eswatini for facilitating the data collection process in-country. September 2019 Table of contents The State of WASH Financing in Eastern and Southern Africa Eswatini Country Level Assessment Table of contents List of abbreviations v 1 Introduction 1 1.1 Background 1 1.2 Methodology 1 1.3 Caveats 2 1.4 Report structure 3 2 Country Context 4 2.1 History/geography 4 2.2 Demography 4 2.3 Macroeconomy 5 2.4 Private Sector Overview 6 2.5 Administrative setup 7 3 WASH Sector Context 8 3.1 Access to WASH Services 8 3.2 Institutional Structures 10 3.3 WASH sector policies, strategies and plans 11 3.4 WASH and Private Sector Involvement 12 4 Government financing of WASH services 13 4.1 Recent Trends 13 4.2 Sector Financing of Strategies and Plans 16 4.3 Framework for donor engagement in the sector 17 5 Donor financing of WASH services 19 5.1 Recent trends 19 5.2 Main Modalities 22 5.3 Coordination of donor support 23 6 Consumer financing of WASH services 24 -

Swaziland Ministry of Agriculture
SWAZILAND MINISTRY OF AGRICULTURE SWAZILAND MARKET ASSESSMENT REPORT DECEMBER 2016 ______________________Shiselweni Region Food Security and Resilience________________________ 0 SWAZILAND MARKET ASSESSMENT REPORT - 2016 Table of Contents List of Figures .....................................................................................................................................2 List of Tables ......................................................................................................................................3 List of Maps .......................................................................................................................................2 Acknowledgments .............................................................................................................................5 Executive summary ............................................................................................................................6 Section 1: Introduction .......................................................................................................................8 1.1 The Economy ............................................................................................................................9 1.2 Food Availability ..................................................................................................................... 11 1.3 Food Security and Nutrition – SwaziVAC 2016.......................................................................... 15 Section 2: Objectives, methodology -

Swaziland Government Gazette Extraordinary
Swaziland Government Gazette Extraordinary VOL. XLVI] MBABANE, Friday, MAY 16th 2008 [No. 67 CONTENTS No. Page PART C - LEGAL NOTICE 104. Registration Centres For the 2008 General Elections................................................... SI PUBLISHED BY AUTHORITY 442 GENERAL NOTICE NO. 25 OF 2008 VOTERS REGISTRATION ORDER, 1992 (King’s Order in Council No.3 of 1992) REGISTRATION CENTRES FOR THE 2008 GENERAL ELECTIONS (Under Section 5(4)) Short title and commencement (1) This notice shall be cited as the Registration Centres Notice, 2008. (2) This general notice shall come into force on the date of publication in the Gazette. Registration centres for the 2008general elections It is notified for general information that the registration of all eligible voters for the 2008 general elections shall be held at Imiphakatsi (chiefdoms) and at the registration centres that have been listed in this notice; REGISTRATION CENTRES HHOHHO REGION CODE CODE CODE CHIEFDOM / POLLING Sub polling REGION INKHUNDLA STATION station 01 HHOHHO 01 HHUKWINI 01 Dlangeni 01 HHOHHO 01 HHUKWINI 02 Lamgabhi 01 HHOHHO 02 LOBAMBA 01 Elangeni 01 HHOHHO 02 LOBAMBA 02 Ezabeni 01 HHOHHO 02 LOBAMBA 03 Ezulwini 01 HHOHHO 02 LOBAMBA 04 Lobamba 01 HHOHHO 02 LOBAMBA 05 Nkhanini 01 HHOHHO 03 MADLANGEMPISI 01 Buhlebuyeza 01 HHOHHO 03 MADLANGEMPISI 02 KaGuquka 01 HHOHHO 03 MADLANGEMPISI 03 Kuphakameni/ Dvokolwako 01 HHOHHO 03 MADLANGEMPISI 04 Mzaceni 01 HHOHHO 03 MADLANGEMPISI 05 Nyonyane / KaMaguga 01 HHOHHO 03 MADLANGEMPISI 06 Zandondo 01 HHOHHO 04 MAPHALALENI 01 Edlozini 443 -

The Kingdom of Swaziland
THE KINGDOM OF SWAZILAND MASTERPLAN TOWARDS THE ELIMINATION OF NEGLECTED TROPICAL DISEASES - 2015- 2020 Foreword Acknowledgements Table of Contents .......................................................................................................................................... 1 LIST OF TABLES .................................................................................................................. 5 PART 1: SITUATION ANALYSIS ....................................................................................... 10 1.1 Country profile ......................................................................................................... 10 1.1.1 Geographical characteristics ............................................................................... 10 1.1 .2 PHYSICAL FEATURES AND CLIMATIC CONDITIONS ....................................... 11 1.1.3. ADMINISTRATIVE STRUCTURES, DEMOGRAPHY AND COMMUNITY STRUCTURES ................................................................................................................... 12 1.3.2 Population ............................................................................................................. 13 Health Information System ........................................................................................... 25 Health workforce ........................................................................................................... 26 Medical products .......................................................................................................... -
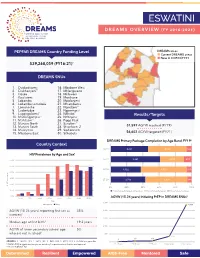
DREAMS Fact Sheet ESWATINI
ESWATINI DREAMS OVERVIEW (fy 2016-2021) PEPFAR DREAMS Country Funding Level DREAMS areas Current DREAMS areas New in COP20/FY21 $39,268,059 (FY16-21)* DREAMS SNUs 1. Dvokodvweni 16. Mbabane West 2. Ekukhanyeni** 17. Mhlangatane 3. Hosea 18. Mkhiweni 4. Kwaluseni 19. Motshane 5. Lobamba 20. Mpolonjeni 6. Lobamba Lomdzala 21. Mtsambama 7. Lomahasha 22. Ngudzeni** 8. Ludzeludze 23. Ngwempisi 9. Lugongolweni** 24. Nkhaba** Results/Targets 10. Madlangampisi** 25. Ntfonjeni 11. Mafutseni** 26. Piggs Peak ** 12. Manzini North 27. Sandleni 5 13. Manzini South 28. Shiselweni 2 31,597 AGYW reached (FY19) 14. Maseyisini 29. Siphofaneni * 15. Mbabane East 30. Sithobela 56,602 AGYW targeted (FY21) DREAMS Primary Package Completion by Age Band, FY195 Country Context 10-14 4,031 4,044 566 HIV Prevalence by Age and Sex1 15-19 60.0% 5,281 2,507 857 50.0% 20-24 3,022 4,515 510 40.0% 30.0% 25-29 2,712 3,208 344 20.0% HIV Prevalence HIV 0% 20% 40% 60% 80% 100% 10.0% Primary Package Completed and Secondary Primary Package Completed Primary Package Incomplete 0.0% 5 10-14 15-19 20-24 25-29 30-34 35-39 40-44 45-49 AGYW (15-24 years) Initiating PrEP in DREAMS SNUs Age 5,000 Female Male 4,755 AGYW (18-24 years) reporting first sex as 35% 4,000 coerced2 3,000 Median age at first birth3 19.2 years 2,000 AGYW of lower secondary school age 5% 4 who are not in school 1,000 704 1 99 108 SOURCES: 1. -

Proceedings of the National Seminar on Institutions Working on Gender, Biodiversity and Local Knowledge Systems in Swaziland
PROCEEDINGS OF THE NATIONAL SEMINAR ON INSTITUTIONS WORKING ON GENDER, BIODIVERSITY AND LOCAL KNOWLEDGE SYSTEMS IN SWAZILAND Edited by Phonius M. Dlamini Department of Agricultural Economics and Management University of Swaziland. E-Mail : [email protected] and Bruce R. T. Vilane Department of Land Use and Mechanization University of Swaziland. E-Mail : [email protected] April, 2001 LinKS PROJECT WORKING DOCUMENT N0. 3 HOSTED BY THE UNIVERSITY OF SWAZILAND – FACULTY OF AGRICULTURE [CONFERENCE ROOM] : 16 – 17 MARCH 2001. COPYRIGHT This document is a collective sharing of ideas and experiences and thus copyright does not reside either with the editors, anyone of the contributors or their parent institutions. However, usage of any material or reproduction, stored in a retrieval system or transmitted in whole or in part in any form or by any means electronic, mechanical, photocopying or otherwise, as a matter of courtesy, acknowledgement of the source should be given. The views expressed are those of the contributors and do not necessarily represent the view of the Food and Agricultural Organization (FAO). Copyright © FAO, 2001. ii TABLE OF CONTENTS Page Copyright ...................................................................................................………………. iii List of Tables .....................................................................................................…………. v Preface.................................................................................................................…………. vi 1.0 KEYNOTE ADDRESS By The Honourable Minister of Tourism, Environment and Communication (Mrs. Stella Lukhele...................…......... 1 2.0 DAY ONE : PAPER PRESENTATIONS……………………………….…… 3 2.1 Gender Papers :…………………………………………………………………….. 3 2.1.1 Gender, Biodiversity and Local Knowledge Systems : Highlights and Submissions of the Seminar Discussions :………………………… 3 2.1.2 Gender and Food Security by Dr. P.E. Zwane :……………………………………. 13 2.2 Biodiversity Papers :.……………………………………………………………… 26 2.2.1 Biodiversity Management in Swaziland by Mr. -

KINGS, COMMONERS and CONCESSIONAIRES the Evolution and Dissolution of the Nineteenth-Century Swazi State AFRICAN STUDIES SERIES
KINGS, COMMONERS AND CONCESSIONAIRES The evolution and dissolution of the nineteenth-century Swazi state AFRICAN STUDIES SERIES 31 Editorial Board John Dunn, Reader in Politics and Fellow of King's College, Cambridge J. M. Lonsdale, Lecturer in History and Fellow of Trinity College, Cambridge D. M. G. Newbery, Lecturer in Economics and Fellow of Churchill College, Cambridge A. F. Robertson, Assistant Director of Development Studies and Fellow of Darwin College, Cambridge The African Studies Series is a collection of monographs and general studies that reflect the interdisciplinary interests of the African Studies Centre at Cambridge. Volumes to date have combined historical, anthropological, economic, political and other perspectives. Each contribution has assumed that such broad approaches can contribute much to our understanding of Africa, and that this may in turn be of advantage to specific disciplines. KINGS, COMMONERS AND CONCESSIONAIRES The Evolution and Dissolution of the Nineteenth-Century Swazi State PHILIP BONNER Senior Lecturer, Department of History University of the Witwatersrand CAMBRIDGE UNIVERSITY PRESS CAMBRIDGE LONDON NEW YORK NEW ROCHELLE MELBOURNE SYDNEY PUBLISHED BY THE PRESS SYNDICATE OF THE UNIVERSITY OF CAMBRIDGE The Pitt Building, Trumpington Street, Cambridge, United Kingdom CAMBRIDGE UNIVERSITY PRESS The Edinburgh Building, Cambridge CB2 2RU, UK 40 West 20th Street, New York NY 10011-4211, USA 477 Williamstown Road, Port Melbourne, VIC 3207, Australia Ruiz de Alarcon 13,28014 Madrid, Spain Dock House, The Waterfront, Cape Town 8001, South Africa http://www.cambridge.org © Cambridge University Press 1982 This book is in copyright. Subject to statutory exception and to the provisions of relevant collective licensing agreements, no reproduction of any part may take place without the written permission of Cambridge University Press. -

SWAZILAND Vulnerability Assessment Committee Results 2015
SWAZILAND Vulnerability Assessment Committee Results 2015 Regional Socio - Economic Context Population at risk of food and livelihoods insecurity trend Malnutrition Rates (%) 2014/15 Population 1,12 million people Stunting Underweight Wasting Life expectancy 47.8 years 262,000 223,249 28.7 31 Population Growth Rate 1.0% 160,989 201,000 29 115,713 Human Development Index 0.148 (2013) 88,511 25.5 Adult Literacy 87.8% (2012) Employment Rate 71.9% (2014) Average GDP Growth 2.3% (2013) 2009/10 2010/11 2011/12 2012/13 2013/14 2014/15 Under 5 Mortality Rate 67 per 1,000 live births May 2015 to April 2016 Projected Livelihood Outcomes 9.6 Inflation 5.70% (2015, CSO) 5.8 HIV and AIDS 26.0% (2009) Timphisini Proportion of Children (%) 5.4 5.8 Ntfonjeni Mayiwane 2.5 2 1.2 0.8 Objectives of Assessment 2014/15 Mhlangatane Pigg's Peak 2000 2007 2010 2014 • To assess the status of livelihoods and vulnerability in rural households and provide timely Ndzingeni information for programming and decision making. 201,000 Lomahasha population at risk of food • To understand the different capabilities (assets) of households to cope with crises such as Mandlangempisi Mhlume Key Recommendations and livelihoods Nkhaba droughts, floods, economic fluctuations, plant or animal pests and diseases. • Crop diversification (not only maize) especially in the Lubombo region and production of insecurity Maphalaleni • Use the Household Economy Approach to get the numbers of people food insecure for the drought resistant crops in this region. consumption period 2015-2016. Mbabane Mkhiweni Hlane • Use of the existing irrigation infrastructure for sugar cane plantations. -

Swaziland ! Bhunya ! ! Inyetane Sandlane ! ! !(Malkerns Magomba ! Usutu Manzini
! 31°0'0"E 31°20'0"E 31°40'0"E 32°0'0"E S S " " 0 0 ' ' 0 0 4 4 ° ° 5 5 2 2 ! Horo ! Ngonini !Barberton o PIGGS PEAK Lomati ! Herefords ! Kobolondo Heights ! Mozambique Rocklands Havelock ! ! ! Bennets Piggs Peak Bulembu ! ! o TJANENI ! Tshaneni Namaacha ! ! Lomahasha S S " " 0 0 ' ' 0 0 ° ° 6 6 2 2 MHo LUME !( South Africa Mhlume Hhohho ! ! Madlangampisi Vuvulane ! Balegane o TAMBANKULU ! Nokwane Forbes Reef ! Mlawula SIMUNYE ! ! Croydon o Ngwenya ! ! Darkton Mliba ! ! Mhlumeni S S " " 0 0 ' .!Mbabane Commissie Nek ' 0 ! 0 2 2 ° ° 6 6 2 Mbuluzana 2 ! Etjanine ! ! Ezulwini ! Skombeni ! Macnab o SITEKI Mhlambanyatsi ! ! Nkanni ! Siteki Kwaluseni! !(Manzini Mahlanya ! MATASAPHA ! Matsapha Luyengo o Inyetane ! !( Swaziland ! Bhunya ! ! Inyetane Sandlane ! ! !(Malkerns Magomba ! Usutu Manzini ! Sidvokodvo S S " Lubombo " 0 0 ' ' 0 0 4 ! 4 ° ° 6 6 2 Mankayane ! Siphofaneni 2 Holobela ! o TAMBUTI Mvangatini ! Nyabe ! Nkonjane ! Singceni ! Stylkloof ! Big Bend ! !( Mgamane ! Abercorn Verdun o SUGAR ESTATE ! Sicunusa ! ! Von Wissel Kubuta Sitobela Rondspring ! ! ! ! Granvalley Gege ! ! ! Mooihoek Hlatikulu S S " " 0 0 ' ' 0 0 ° ° 7 ! 7 2 Piet Retief Lubuli 2 Maloma ! ! Shiselweni Nsoko ! NSOKO o Mahamba ! Lismore Nhlangano!(o ! NHLANGANO Dwaleni ! Mhlosheni ! Hluti! ! S S " Lavumisa " 0 0 ' ' 0 0 2 2 ° ° 7 7 2 2 31°0'0"E 31°20'0"E 31°40'0"E 32°0'0"E National Capital Primary road National boundary .! Date Created: 19 - APR -2013 (! Major Town Secondary road First level admin Map Num: SWZ_GLPM_A2P boundary Coord.System/Datum: Geographic/WGS84 GLIDE Num: S W A Z I L A N D !( Intermediate Town Tertiary road River Data Sources: WFP, GLCSC, ESRI, UNGIWG, ! Small Town Track/Trail Geonames General Logistics Planning Map Surface Waterbody ! Village Railway The boundaries and names and the designations used on this map do not imply official endorsement or acceptance by the United Nations.