Ethanol's Action at BK Channels Accelerates the Transition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evaluation of Perennial Ryegrass Straw As a Forage Source for Ruminants
AN ABSTRACT OF THE THESIS OF Michael J. Fisher for the degree of Master of Science in Animal Sciencepresented on July 28, 2003. Title: Evaluation of Perennial Ryegrass Strawas a Forage Source for Ruminants. Redacted for Privacy Abstract approved: David W. Bohnert We conducted two experiments evaluating perennialryegrass straw as a forage source for ruminants. Experiment 1 evaluated digestion and physiological variables in steers offered perennial ryegrass straw containing increasing levels of lolitrem B. Sixteen ruminally cannulated Angus X Hereford steers (231 ± 2 kg BW)were blocked by weight and assigned randomly to one of four treatments (TRT). Steerswere provided perennial ryegrass straw at 120% of the previous 5-daverage intake. Prior to straw feeding, soybean meal (SBM) was provided (0.1% BW; CP basis) tomeet the estimated requirement for degradable intake protein. Low (L) and high(H) lolitrem B straws (<100 and 1550 ppb, respectively) were used to formulate TRT diets: LOW (100% L); LOW MIX (67% L:33% H); HIGH MIX (33% L:67% H); HIGH(100% H). Intake and digestibility of DM and OM, and ruminal pH, total VFA, andNH3-N were not affected by increasing lolitrem B concentration (P> 0.13). Ruminal indigestible ADF (IADF) fill increased linearly (P= 0.01) and JADF passage rate (%/h) decreased linearly (P = 0.04) as lolitrem B level increased. Experiment2 evaluated performance and production of 72 Angus X Herefordcows (539 ± 5 kg BW) consuming perennial ryegrass straw containing increasing levels of lolitremB during the last third of gestation. Cowswere blocked by body condition score (BCS) and randomly assigned to one of three TRT. -

Interactions of Insecticidal Spider Peptide Neurotoxins with Insect Voltage- and Neurotransmitter-Gated Ion Channels
Interactions of insecticidal spider peptide neurotoxins with insect voltage- and neurotransmitter-gated ion channels (Molecular representation of - HXTX-Hv1c including key binding residues, adapted from Gunning et al, 2008) PhD Thesis Monique J. Windley UTS 2012 CERTIFICATE OF AUTHORSHIP/ORIGINALITY I certify that the work in this thesis has not previously been submitted for a degree nor has it been submitted as part of requirements for a degree except as fully acknowledged within the text. I also certify that the thesis has been written by me. Any help that I have received in my research work and the preparation of the thesis itself has been acknowledged. In addition, I certify that all information sources and literature used are indicated in the thesis. Monique J. Windley 2012 ii ACKNOWLEDGEMENTS There are many people who I would like to thank for contributions made towards the completion of this thesis. Firstly, I would like to thank my supervisor Prof. Graham Nicholson for his guidance and persistence throughout this project. I would like to acknowledge his invaluable advice, encouragement and his neverending determination to find a solution to any problem. He has been a valuable mentor and has contributed immensely to the success of this project. Next I would like to thank everyone at UTS who assisted in the advancement of this research. Firstly, I would like to acknowledge Phil Laurance for his assistance in the repair and modification of laboratory equipment. To all the laboratory and technical staff, particulary Harry Simpson and Stan Yiu for the restoration and sourcing of equipment - thankyou. I would like to thank Dr Mike Johnson for his continual assistance, advice and cheerful disposition. -
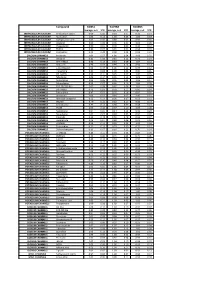
Biomol Average and SD Table S1.Xlsx
Compound GliNS1 G179NS G166NS average, n=5 STD average, n=3 STD average, n=3 STD INTRACELLULAR CALCIUM Antibiotic A-23187 0.00 0.00 0.10 0.07 0.15 0.14 INTRACELLULAR CALCIUM Ryanodine 1.04 0.14 1.03 0.03 1.03 0.03 INTRACELLULAR CALCIUM Cyclopiazonic acid 1.01 0.06 0.88 0.05 0.92 0.06 INTRACELLULAR CALCIUM Gingerol 1.00 0.06 0.91 0.01 1.01 0.06 INTRACELLULAR CALCIUM Thapsigargin 0.00 0.01 0.00 0.00 0.10 0.12 INTRACELLULAR CALCIUM TMB-8 0.89 0.07 0.91 0.05 0.94 0.03 INTRACELLULAR CALCIUM Dantrolene 0.91 0.08 0.98 0.05 0.94 0.01 CALCIUM CHANNELS Amiloride 1.01 0.07 1.01 0.04 1.03 0.05 CALCIUM CHANNELS Benzamil 0.83 0.08 0.83 0.12 0.96 0.04 CALCIUM CHANNELS BAY K-8644 0.93 0.13 0.93 0.09 1.07 0.14 CALCIUM CHANNELS Diltiazem 0.96 0.07 0.99 0.12 0.94 0.14 CALCIUM CHANNELS L-cis-Diltiazem 0.91 0.17 1.01 0.12 0.95 0.12 CALCIUM CHANNELS Flunarizine 0.85 0.08 1.00 0.06 0.85 0.05 CALCIUM CHANNELS FPL-64176 0.99 0.11 0.95 0.07 1.05 0.05 CALCIUM CHANNELS Nifedipine 1.06 0.17 0.95 0.12 1.03 0.09 CALCIUM CHANNELS Nimodipine 1.05 0.06 0.95 0.03 1.06 0.17 CALCIUM CHANNELS Nitrendipine 0.99 0.07 0.96 0.10 1.04 0.09 CALCIUM CHANNELS SDZ-202791 R(-) 1.01 0.08 0.92 0.06 1.01 0.08 CALCIUM CHANNELS SKF-96365 0.73 0.05 0.70 0.11 0.69 0.04 CALCIUM CHANNELS Tetrandrine 0.47 0.07 0.76 0.16 0.87 0.20 CALCIUM CHANNELS Verapamil 1.01 0.02 0.89 0.07 1.06 0.20 CALCIUM CHANNELS Methoxy Verapamil 0.93 0.14 0.96 0.07 0.93 0.13 CALCIUM CHANNELS Bepridil 0.70 0.16 0.92 0.15 0.84 0.14 CALCIUM CHANNELS Amiodarone 0.32 0.12 0.58 0.07 0.48 0.23 CALCIUM CHANNELS YS035 1.00 0.16 -

Penitrem and Thomitrem Formation by Penicillium Crustosum
Mycopathologia 157: 349–357, 2004. 349 © 2004 Kluwer Academic Publishers. Printed in the Netherlands. Penitrem and thomitrem formation by Penicillium crustosum Thomas Rundberget1, Ida Skaar1, Oloff O’Brien2 & Arne Flåøyen1 1National Veterinary Institute, PO Box 8156 Dep., 0033 Oslo, Norway; 2ARC Plant Protection, Research Institute, Private Bag X134, Pretoria 0001, South Africa Received 9 September 2002; accepted in final form 16 July 2003 Abstract The levels of penitrems A, B, C, D, E, F, roquefortine C and thomitrem A and E recovered from extracts of 36 Norwegian, 2 American and one each of Japanese, German, South African, Danish and Fijian isolates of Penicillum crustosum Thom were quantitatively determined using high performance liquid chromatography-mass spectrometry (HPLC-MS). Forty-two of the 44 isolates of penitrem-producing isolates grown on rice, afforded levels of thomitrem A and E comparable to that of penitrem A. Thomitrems A and E were also found, but at lower levels, when cultures were grown on barley. No thomitrems were found when the isolates were grown on liquid media. The effects of time and temperature on mycotoxin formation were studied on rice over a 4 week period at 10, 15 and 25 ◦C, respectively. No mycotoxins could be detected after 1 week at 10 ◦C, but after 2 weeks at 10 ◦C levels were similar to those produced at 15 and 25 ◦C. Higher levels of thomitrems A and E were detected when media were maintained at lower pH. The possibility that thomitrems A and E might be derived by acid promoted conversion of penitrems A and E was explored in stability trials performed at pH 2, 3, 4, 5 and 7 in the presence and absence of media. -

(BK) Channel Antagonist Penitrem a As a Novel Breast Cancer-Targeted Therapeutic
marine drugs Article The Maxi-K (BK) Channel Antagonist Penitrem A as a Novel Breast Cancer-Targeted Therapeutic Amira A. Goda 1, Abu Bakar Siddique 1 ID , Mohamed Mohyeldin 1,3, Nehad M. Ayoub 2 ID and Khalid A. El Sayed 1,* ID 1 Department of Basic Pharmaceutical Sciences, School of Pharmacy, University of Louisiana at Monroe, 1800 Bienville Drive, Monroe, LA 71201, USA; [email protected] (A.A.G.); [email protected] (A.B.S.); [email protected] (M.M.) 2 Department of Clinical Pharmacy, Faculty of Pharmacy, Jordan University of Science and Technology, Irbid 22110, Jordan; [email protected] 3 Department of Pharmacognosy, Faculty of Pharmacy, Alexandria University, Alexandria 21521, Egypt * Correspondence: [email protected]; Tel.: +1-318-342-1725 Received: 6 April 2018; Accepted: 9 May 2018; Published: 11 May 2018 Abstract: Breast cancer (BC) is a heterogeneous disease with different molecular subtypes. The high conductance calcium-activated potassium channels (BK, Maxi-K channels) play an important role in the survival of some BC phenotypes, via membrane hyperpolarization and regulation of cell cycle. BK channels have been implicated in BC cell proliferation and invasion. Penitrems are indole diterpene alkaloids produced by various terrestrial and marine Penicillium species. Penitrem A (1) is a selective BK channel antagonist with reported antiproliferative and anti-invasive activities against multiple malignancies, including BC. This study reports the high expression of BK channel in different BC subtypes. In silico BK channel binding affinity correlates with the antiproliferative activities of selected penitrem analogs. 1 showed the best binding fitting at multiple BK channel crystal structures, targeting the calcium-sensing aspartic acid moieties at the calcium bowel and calcium binding sites. -

Veterinary Toxicology
GINTARAS DAUNORAS VETERINARY TOXICOLOGY Lecture notes and classes works Study kit for LUHS Veterinary Faculty Foreign Students LSMU LEIDYBOS NAMAI, KAUNAS 2012 Lietuvos sveikatos moksl ų universitetas Veterinarijos akademija Neužkre čiam ųjų lig ų katedra Gintaras Daunoras VETERINARIN Ė TOKSIKOLOGIJA Paskait ų konspektai ir praktikos darb ų aprašai Mokomoji knyga LSMU Veterinarijos fakulteto užsienio studentams LSMU LEIDYBOS NAMAI, KAUNAS 2012 UDK Dau Apsvarstyta: LSMU VA Veterinarijos fakulteto Neužkre čiam ųjų lig ų katedros pos ėdyje, 2012 m. rugs ėjo 20 d., protokolo Nr. 01 LSMU VA Veterinarijos fakulteto tarybos pos ėdyje, 2012 m. rugs ėjo 28 d., protokolo Nr. 08 Recenzavo: doc. dr. Alius Pockevi čius LSMU VA Užkre čiam ųjų lig ų katedra dr. Aidas Grigonis LSMU VA Neužkre čiam ųjų lig ų katedra CONTENTS Introduction ……………………………………………………………………………………… 7 SECTION I. Lecture notes ………………………………………………………………………. 8 1. GENERAL VETERINARY TOXICOLOGY ……….……………………………………….. 8 1.1. Veterinary toxicology aims and tasks ……………………………………………………... 8 1.2. EC and Lithuanian legal documents for hazardous substances and pollution ……………. 11 1.3. Classification of poisons ……………………………………………………………………. 12 1.4. Chemicals classification and labelling ……………………………………………………… 14 2. Toxicokinetics ………………………………………………………………………...………. 15 2.2. Migration of substances through biological membranes …………………………………… 15 2.3. ADME notion ………………………………………………………………………………. 15 2.4. Possibilities of poisons entering into an animal body and methods of absorption ……… 16 2.5. Poison distribution -

Tremorgenic Mycotoxin Intoxication by Mary M
Tremorgenic mycotoxin intoxication by Mary M. Schell, DVM Dogs allowed to roam or get into the trash may ingest tremorgenic mycotoxins, which are neorotoxins that produce varying degrees of muscle tremors or seizures that can last for hours or days. Since 1998, the ASPCA Animal Poison Control Center (APCC) has consulted on 25 cases of suspected tremorgenic mycotoxin intoxication in dogs and one in a squirrel. Sources of tremorgenic mycotoxins for household pets have included moldy dairy foods, moldy walnuts or peanuts, stored grains, and moldy spaghetti. 1-7 These toxic secondary metabolites of many fungi vary in quantity and in their ability to produce clinical effects. Toxin production depends on seasonal growing conditions as well as the genus and species of the mold. At least 20 mycotoxins have been identified as tremorgens (compounds capable of inducing serious muscle tremor in one or more vertebrates), although only a few have been shown to have clinical relevance.3,8 Penicillium species are most often incriminated in producing tremorgenic mycotoxins; the most common are penitrem-A and roquefortine C.1,3,6,8 Intoxication with these mycotoxins has been documented in many animals, including dogs, cattle, sheep, rabbits, poultry, and rodents. Several mechanisms of action have been proposed, and the mechanism may vary both between toxins and the individual susceptible species. Penitrem-A inhibits the inhibitory neurotransmitter glycine in mice. Studies in mice have shown that drugs such as mephenesin or nalorphine, which increase glycine -

Glycine311, a Determinant of Paxilline Block in BK Channels: a Novel Bend in the BK S6 Helix Yu Zhou Washington University School of Medicine in St
Washington University School of Medicine Digital Commons@Becker Open Access Publications 2010 Glycine311, a determinant of paxilline block in BK channels: A novel bend in the BK S6 helix Yu Zhou Washington University School of Medicine in St. Louis Qiong-Yao Tang Washington University School of Medicine in St. Louis Xiao-Ming Xia Washington University School of Medicine in St. Louis Christopher J. Lingle Washington University School of Medicine in St. Louis Follow this and additional works at: http://digitalcommons.wustl.edu/open_access_pubs Recommended Citation Zhou, Yu; Tang, Qiong-Yao; Xia, Xiao-Ming; and Lingle, Christopher J., ,"Glycine311, a determinant of paxilline block in BK channels: A novel bend in the BK S6 helix." Journal of General Physiology.135,5. 481-494. (2010). http://digitalcommons.wustl.edu/open_access_pubs/2878 This Open Access Publication is brought to you for free and open access by Digital Commons@Becker. It has been accepted for inclusion in Open Access Publications by an authorized administrator of Digital Commons@Becker. For more information, please contact [email protected]. Published April 26, 2010 A r t i c l e Glycine311, a determinant of paxilline block in BK channels: a novel bend in the BK S6 helix Yu Zhou, Qiong-Yao Tang, Xiao-Ming Xia, and Christopher J. Lingle Department of Anesthesiology, Washington University School of Medicine, St. Louis, MO 63110 The tremorogenic fungal metabolite, paxilline, is widely used as a potent and relatively specific blocker of Ca2+- and voltage-activated Slo1 (or BK) K+ channels. The pH-regulated Slo3 K+ channel, a Slo1 homologue, is resistant to blockade by paxilline. -

Penitrem a Peer-Reviewed Journal Articles
________________________________________________________________________ ________________________________________________________________________ Penitrem A Peer-Reviewed Journal Articles Literature Review by Lisa Petrison, Ph.D. Neurotoxic Effects: Berntsen H. F., Wigestrand M. B., Bogen I. L., Fonnum F., Walaas S. I., Moldes-Anaya A.. Mechanisms of penitrem-induced cerebellar granule neuron death in vitro: possible involvement of GABAA receptors and oxidative processes. Neurotoxicology. 2013;35:129–136. Moldes-Anaya Angel, Rundberget Thomas, Fæste Christiane K., Eriksen Gunnar S., Bernhoft Aksel. Neurotoxicity of Penicillium crustosum secondary metabolites: tremorgenic activity of orally administered penitrem A and thomitrem A and E in mice. Toxicon. 2012;60:1428–1435. Moldes-Anaya Angel S., Fonnum Frode, Eriksen Gunnar S., Rundberget Thomas, Walaas S. Ivar, Wigestrand Mattis B.. In vitro neuropharmacological evaluation of penitrem-induced tremorgenic syndromes: importance of the GABAergic system. Neurochemistry international. 2011;59:1074–1081. Lu Hai-Xia X., Levis Hannah, Liu Yong, Parker Terry. Organotypic slices culture model for cerebellar ataxia: potential use to study Purkinje cell induction from neural stem cells. Brain research bulletin. 2011;84:169–173. Namiranian Khodadad, Lloyd Eric E., Crossland Randy F., et al. Cerebrovascular responses in mice deficient in the potassium channel, TREK-1. American journal of physiology. Regulatory, integrative and comparative physiology. 2010;299. Ohno Akitoshi, Ohya Susumu, Yamamura Hisao, -

Tremorgenic Mycotoxins: Structure Diversity and Biological Activity
toxins Review Tremorgenic Mycotoxins: Structure Diversity and Biological Activity Priyanka Reddy 1,2, Kathryn Guthridge 1, Simone Vassiliadis 1 , Joanne Hemsworth 1, Inoka Hettiarachchige 1, German Spangenberg 1,2 and Simone Rochfort 1,2,* 1 Agriculture Victoria, AgriBio, Centre for AgriBioscience, Bundoora, Victoria 3083, Australia; [email protected] (P.R.); [email protected] (K.G.); [email protected] (S.V.); [email protected] (J.H.); [email protected] (I.H.); [email protected] (G.S.) 2 School of Applied Systems Biology, La Trobe University, Bundoora, Victoria 3083, Australia * Correspondence: [email protected] Received: 24 April 2019; Accepted: 22 May 2019; Published: 27 May 2019 Abstract: Indole-diterpenes are an important class of chemical compounds which can be unique to different fungal species. The highly complex lolitrem compounds are confined to Epichloë species, whilst penitrem production is confined to Penicillium spp. and Aspergillus spp. These fungal species are often present in association with pasture grasses, and the indole-diterpenes produced may cause toxicity in grazing animals. In this review, we highlight the unique structural variations of indole-diterpenes that are characterised into subgroups, including paspaline, paxilline, shearinines, paspalitrems, terpendoles, penitrems, lolitrems, janthitrems, and sulpinines. A detailed description of the unique biological activities has been documented where even structurally related compounds have displayed unique biological activities. Indole-diterpene production has been reported in two classes of ascomycete fungi, namely Eurotiomycetes (e.g., Aspergillus and Penicillium) and Sordariomycetes (e.g., Claviceps and Epichloë). -

Big Conductance Calcium-Activated Potassium Channel Openers Control Spasticity Without Sedation
British Journal of British Journal of Pharmacology (2017) 174 2662–2681 2662 BJP Pharmacology RESEARCH PAPER Big conductance calcium-activated potassium channel openers control spasticity without sedation Correspondence Professor David Baker, Neuroimmunology Unit, Blizard Institute, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, 4 Newark Street, Whitechapel, London E1 4AT, UK. E-mail: [email protected] Received 5 January 2017; Revised 27 April 2017; Accepted 17 May 2017 David Baker1,2,* ,GarethPryce1,2,*, Cristina Visintin2,3,SofiaSisay1, Alexander I Bondarenko4,5, WSVanessaHo6, Samuel J Jackson1, Thomas E Williams1, Sarah Al-Izki1, Ioanna Sevastou3, Masahiro Okuyama3, Wolfgang F Graier4, Lesley A Stevenson6,CarolynTanner7,RuthRoss7, Roger G Pertwee7, Christopher M Henstridge8,AndrewJIrving8, Jesse Schulman9,KeithPowell9,MarkDBaker1, Gavin Giovannoni1,2 and David L Selwood3,* 1Neuroimmunology Unit, Blizard Institute, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London, UK, 2Department of Neuroinflammation, UCL Institute of Neurology, University College London, London, UK, 3Department of Medicinal Chemistry, UCL Wolfson Institute for Biomedical Research, University College London, London, UK, 4Institute of Molecular Biology and Biochemistry, Medical University of Graz, Graz, Austria, 5A.A. Bogomoletz Institute of Physiology, Kiev, Ukraine, 6Vascular Biology Research Centre. St. George’s, University of London, London, UK, 7Department of Biomedical Sciences, -

Functional Analysis of Penicillium Paxilli Genes Required for Biosynthesis of Paxilline
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere without the permission of the Author. Functional analysis of Penicillium paxilli genes required for biosynthesis of paxilline This thesis is presented in partial fulfilment of the requirements for the degree of Doctor of Philosophy (PhD) In Biochemistry at Massey University, Palmerston North, New Zealand Sanjay Saikia 2006 ABSTRACT Paxilline belongs to a large, structurally and functionally diverse group of indole-diterpenes and is synthesised by the filamentous fungus Penicillium paxilli. A gene cluster for paxilline biosynthesis in P. paxilli has been identified and characterised. However, none of the steps proposed in the biosynthesis of paxilline or paxilline-like indole-diterpenes have been validated . In some diterpene-producing fi lamentous fungi, including P. paxilli, two distinct copies of geranylgeranyl diphosphate (GGPP) synthase, that catalyses the committed step in diterpene biosynthesis, have been identified . However, the biological significance of the presence of two distinct GGPP synthases is not known. In this study, biochemical analysis of the paxilline gene products in P. paxilli and subcellular localisation of the two P. paxilli GGPP synthases, Ggs1 and PaxG, were carried out. Transfer of constructs containing different combinations of pax genes into a pax cluster negative deletion derivative of P. paxilli identified four Pax proteins that are required for the biosynthesis of a paxilline intermediate, paspaline. These proteins are PaxG, a GGPP synthase, PaxM, a FAD-dependent monooxygenase, PaxB, a putative membrane protein, and PaxC, a prenyltransferase.