Hypercholesterolemia Effect on Potassium Channels
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
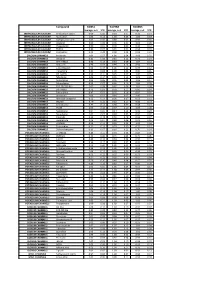
Biomol Average and SD Table S1.Xlsx
Compound GliNS1 G179NS G166NS average, n=5 STD average, n=3 STD average, n=3 STD INTRACELLULAR CALCIUM Antibiotic A-23187 0.00 0.00 0.10 0.07 0.15 0.14 INTRACELLULAR CALCIUM Ryanodine 1.04 0.14 1.03 0.03 1.03 0.03 INTRACELLULAR CALCIUM Cyclopiazonic acid 1.01 0.06 0.88 0.05 0.92 0.06 INTRACELLULAR CALCIUM Gingerol 1.00 0.06 0.91 0.01 1.01 0.06 INTRACELLULAR CALCIUM Thapsigargin 0.00 0.01 0.00 0.00 0.10 0.12 INTRACELLULAR CALCIUM TMB-8 0.89 0.07 0.91 0.05 0.94 0.03 INTRACELLULAR CALCIUM Dantrolene 0.91 0.08 0.98 0.05 0.94 0.01 CALCIUM CHANNELS Amiloride 1.01 0.07 1.01 0.04 1.03 0.05 CALCIUM CHANNELS Benzamil 0.83 0.08 0.83 0.12 0.96 0.04 CALCIUM CHANNELS BAY K-8644 0.93 0.13 0.93 0.09 1.07 0.14 CALCIUM CHANNELS Diltiazem 0.96 0.07 0.99 0.12 0.94 0.14 CALCIUM CHANNELS L-cis-Diltiazem 0.91 0.17 1.01 0.12 0.95 0.12 CALCIUM CHANNELS Flunarizine 0.85 0.08 1.00 0.06 0.85 0.05 CALCIUM CHANNELS FPL-64176 0.99 0.11 0.95 0.07 1.05 0.05 CALCIUM CHANNELS Nifedipine 1.06 0.17 0.95 0.12 1.03 0.09 CALCIUM CHANNELS Nimodipine 1.05 0.06 0.95 0.03 1.06 0.17 CALCIUM CHANNELS Nitrendipine 0.99 0.07 0.96 0.10 1.04 0.09 CALCIUM CHANNELS SDZ-202791 R(-) 1.01 0.08 0.92 0.06 1.01 0.08 CALCIUM CHANNELS SKF-96365 0.73 0.05 0.70 0.11 0.69 0.04 CALCIUM CHANNELS Tetrandrine 0.47 0.07 0.76 0.16 0.87 0.20 CALCIUM CHANNELS Verapamil 1.01 0.02 0.89 0.07 1.06 0.20 CALCIUM CHANNELS Methoxy Verapamil 0.93 0.14 0.96 0.07 0.93 0.13 CALCIUM CHANNELS Bepridil 0.70 0.16 0.92 0.15 0.84 0.14 CALCIUM CHANNELS Amiodarone 0.32 0.12 0.58 0.07 0.48 0.23 CALCIUM CHANNELS YS035 1.00 0.16 -

Pain Research Product Guide | Edition 2
Pain Research Product Guide | Edition 2 Chili plant Capsicum annuum A source of Capsaicin Contents by Research Area: • Nociception • Ion Channels • G-Protein-Coupled Receptors • Intracellular Signaling Tocris Product Guide Series Pain Research Contents Page Nociception 3 Ion Channels 4 G-Protein-Coupled Receptors 12 Intracellular Signaling 18 List of Acronyms 21 Related Literature 22 Pain Research Products 23 Further Reading 34 Introduction Pain is a major public health problem with studies suggesting one fifth of the general population in both the USA and Europe are affected by long term pain. The International Association for the Study of Pain (IASP) defines pain as ‘an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage’. Management of chronic pain in the clinic has seen only limited progress in recent decades. Treatment of pain has been reliant on, and is still dominated by two classical medications: opioids and non-steroidal anti-inflammatory drugs (NSAIDs). However, side effects such as dependence associated with opioids and gastric ulceration associated with NSAIDs demonstrates the need for new drug targets and novel compounds that will bring in a new era of pain therapeutics. Pain has been classified into three major types: nociceptive pain, inflammatory pain and neuropathic or pathological pain. Nociceptive pain involves the transduction of painful stimuli by peripheral sensory nerve fibers called nociceptors. Neuropathic pain results from damage or disease affecting the sensory system, and inflammatory pain represents the immunological response to injury through inflammatory mediators that contribute to pain. Our latest pain research guide focuses on nociception and the transduction of pain to the spinal cord, examining some of the main classical targets as well as emerging pain targets. -

Structural and Biochemical Studies of the Human Two Pore Domain Potassium Channel K2P1
STRUCTURAL AND BIOCHEMICAL STUDIES OF THE HUMAN TWO PORE DOMAIN POTASSIUM CHANNEL K2Pl by Alexandria Nuesa Miller A Dissertation Presented to the Faculty ofthe Louis V. Gerstner, Jr. Graduate School of Biomedical Sciences, Memorial Sloan-Kettering Cancer Center in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy New York, NY April, 2013 ~y;} c;, ZD/3 s~ Date Dissertation Mentor Copyright by Alexandria N. Miller 2013 ABSTRACT Potassium (K+) channels are the largest family of ion channels in eukaryotes with over 70 genes in humans. They have diverse functional roles including controlling the firing duration and frequency of actions potentials in neurons and regulating water retention in the kidneys. K+ channels are highly-selective for K+ over other monovalent cations, can conduct K+ at rates approaching 108 ions per second and, like other ion channels, switch between conductive (open) and non-conductive (closed) states through a process called gating. + Two pore domain (K2P) potassium channels, originally called K background or leak channels, represent a subclass of K+ channels that function to establish and maintain the resting potential in eukaryotic cells. This process primes cells for diverse responses such as action potentials in excitatory cells and cell signaling cascades, which can direct growth and motility in non-excitable cell types. K2P channels have been shown to be gated by a range of cell stimuli and pharmacological agents including temperature, pH, polyunsaturated fatty acids, mechanical stress, and anesthetics. Not surprisingly, it is proposed that they are involved in physiological processes such as pain perception and anesthetic modulation. Structural studies of K2P channels would not only provide insight into how K2P channels are gated by these stimuli, but also may suggest strategies for the generation of K2P specific drugs. -

Ion Channels
UC Davis UC Davis Previously Published Works Title THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Ion channels. Permalink https://escholarship.org/uc/item/1442g5hg Journal British journal of pharmacology, 176 Suppl 1(S1) ISSN 0007-1188 Authors Alexander, Stephen PH Mathie, Alistair Peters, John A et al. Publication Date 2019-12-01 DOI 10.1111/bph.14749 License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology (2019) 176, S142–S228 THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Ion channels Stephen PH Alexander1 , Alistair Mathie2 ,JohnAPeters3 , Emma L Veale2 , Jörg Striessnig4 , Eamonn Kelly5, Jane F Armstrong6 , Elena Faccenda6 ,SimonDHarding6 ,AdamJPawson6 , Joanna L Sharman6 , Christopher Southan6 , Jamie A Davies6 and CGTP Collaborators 1School of Life Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK 2Medway School of Pharmacy, The Universities of Greenwich and Kent at Medway, Anson Building, Central Avenue, Chatham Maritime, Chatham, Kent, ME4 4TB, UK 3Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK 4Pharmacology and Toxicology, Institute of Pharmacy, University of Innsbruck, A-6020 Innsbruck, Austria 5School of Physiology, Pharmacology and Neuroscience, University of Bristol, Bristol, BS8 1TD, UK 6Centre for Discovery Brain Science, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2019/20 is the fourth in this series of biennial publications. The Concise Guide provides concise overviews of the key properties of nearly 1800 human drug targets with an emphasis on selective pharmacology (where available), plus links to the open access knowledgebase source of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. -

Inward Rectifier Potassium Current in Dopaminergic Periglomerular Cells of Mouse Olfactory Bulb
UNIVERSITY OF INSUBRIA Varese A Thesis submitted for the degree of Philosophiæ Doctor (Ph.D.) in Experimental and Clinical Physiology XXVI cycle Inward Rectifier Potassium Current in Dopaminergic Periglomerular Cells of Mouse Olfactory Bulb Tutor Dr Elena Bossi Co-tutor Prof. Ottorino Belluzzi Coordinator Prof. Daniela Negrini Ph.D. Student Dr. Mirta Borin Academic Year 2012-2013 CONTENTS Abbreviations and Acronyms Abstract Chapter 1 INTRODUCTION 1.1 The Main Olfactory System 3 1.1.1 Neuronal Replacement in the Adult Olfactory System 5 1.2 Olfactory Bulb 7 1.2.1 Synaptic Processing within the Olfactory Bulb 8 1.3 Periglomerular Cells 11 1.3.1 TH- and GABA-positive JG cells 13 1.3.2 Electrophysiological Characterisation of TH-positive PG Cells 14 1.4 Kir Channels 16 1.4.1 Architecture of Kir channels 17 1.4.2 Ion selectivity 19 1.4.3 Inward rectification 20 1.4.4 Cytoplasmic regulatory factor 24 1.5 Inward rectifier family members 29 1.5.1 Classical Kir channels 29 1.5.2 G-protein gated Kir channels (GIRK) 30 1.5.3 ATP-sensitive K+ channels 32 1.5.4 K+-transport channels 34 Chapter 2 MATERIAL AND METHODS 2.1 Animals 36 2.2 Slice Preparation 36 2.3 Recording Condition 38 2.4 Electrophysiological Experimental Set-up 40 2.5 Solutions 41 2.6 Analysis of Current Recordings 42 2.7 Statistical Analysis 43 Chapter 3 RESULTS 3.1 Identification and Basic Properties 44 3.1.1 Hyperpolarizing Step: Two Current Components 44 3.1.2 Barium Sensitivity 46 3.1.3 Ba2+ and Cs+ Voltage Dependent Block 47 3.1.4 Potassium and Voltage Dependence of IKir 48 3.1.5 -

Big Conductance Calcium-Activated Potassium Channel Openers Control Spasticity Without Sedation
British Journal of British Journal of Pharmacology (2017) 174 2662–2681 2662 BJP Pharmacology RESEARCH PAPER Big conductance calcium-activated potassium channel openers control spasticity without sedation Correspondence Professor David Baker, Neuroimmunology Unit, Blizard Institute, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, 4 Newark Street, Whitechapel, London E1 4AT, UK. E-mail: [email protected] Received 5 January 2017; Revised 27 April 2017; Accepted 17 May 2017 David Baker1,2,* ,GarethPryce1,2,*, Cristina Visintin2,3,SofiaSisay1, Alexander I Bondarenko4,5, WSVanessaHo6, Samuel J Jackson1, Thomas E Williams1, Sarah Al-Izki1, Ioanna Sevastou3, Masahiro Okuyama3, Wolfgang F Graier4, Lesley A Stevenson6,CarolynTanner7,RuthRoss7, Roger G Pertwee7, Christopher M Henstridge8,AndrewJIrving8, Jesse Schulman9,KeithPowell9,MarkDBaker1, Gavin Giovannoni1,2 and David L Selwood3,* 1Neuroimmunology Unit, Blizard Institute, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London, UK, 2Department of Neuroinflammation, UCL Institute of Neurology, University College London, London, UK, 3Department of Medicinal Chemistry, UCL Wolfson Institute for Biomedical Research, University College London, London, UK, 4Institute of Molecular Biology and Biochemistry, Medical University of Graz, Graz, Austria, 5A.A. Bogomoletz Institute of Physiology, Kiev, Ukraine, 6Vascular Biology Research Centre. St. George’s, University of London, London, UK, 7Department of Biomedical Sciences, -

Functional Analysis of Penicillium Paxilli Genes Required for Biosynthesis of Paxilline
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere without the permission of the Author. Functional analysis of Penicillium paxilli genes required for biosynthesis of paxilline This thesis is presented in partial fulfilment of the requirements for the degree of Doctor of Philosophy (PhD) In Biochemistry at Massey University, Palmerston North, New Zealand Sanjay Saikia 2006 ABSTRACT Paxilline belongs to a large, structurally and functionally diverse group of indole-diterpenes and is synthesised by the filamentous fungus Penicillium paxilli. A gene cluster for paxilline biosynthesis in P. paxilli has been identified and characterised. However, none of the steps proposed in the biosynthesis of paxilline or paxilline-like indole-diterpenes have been validated . In some diterpene-producing fi lamentous fungi, including P. paxilli, two distinct copies of geranylgeranyl diphosphate (GGPP) synthase, that catalyses the committed step in diterpene biosynthesis, have been identified . However, the biological significance of the presence of two distinct GGPP synthases is not known. In this study, biochemical analysis of the paxilline gene products in P. paxilli and subcellular localisation of the two P. paxilli GGPP synthases, Ggs1 and PaxG, were carried out. Transfer of constructs containing different combinations of pax genes into a pax cluster negative deletion derivative of P. paxilli identified four Pax proteins that are required for the biosynthesis of a paxilline intermediate, paspaline. These proteins are PaxG, a GGPP synthase, PaxM, a FAD-dependent monooxygenase, PaxB, a putative membrane protein, and PaxC, a prenyltransferase. -

Correlation of Fecal Ergovaline, Lolitrem B, and Their Metabolites in Steers Fed Endophyte Infected Perennial Ryegrass Straw
AN ABSTRACT OF THE THESIS OF Lia D. Murty for the degree of Master of Science in Pharmacy presented on November 21, 2012. Title: Correlation of Fecal Ergovaline, Lolitrem B, and their Metabolites in Steers Fed Endophyte Infected Perennial Ryegrass Straw Abstract approved: A. Morrie Craig Perennial ryegrass (PRG, Lolium perenne) is a hardy cool-season grass that is infected with the endophytic fungus Neotyphodium lolii, which enables the plant to be insect repellant and drought resistant, lowering the use of insecticides and fertilizers. However, this fungus produces the compound lolitrem B (LB, m/z 686.4) which causes the tremorgenic neurotoxicity syndrome ‘ryegrass staggers’ in livestock consuming forage which contains <2000 ppb LB. Ergovaline (EV, m/z 534) is a vasoconstrictor normally associated with tall fescue (Festuca arudinacea), but has also been found in endophyte- infected PRG. Past research has shown a strong linear correlation between levels of LB and EV in PRG. The purpose of this study was to examine the linear relationship between EV and LB in feces and determine common metabolites. To accomplish this, four groups of steers (n=6/group) consumed endophyte- infected PRG over 70 days consumed the following averages of LB and EV: group I 2254ppb LB/633 ppb EV; group II 1554ppb LB/ 373ppb EV, group III 1011ppb LB/259ppb EV, and group IV 246ppb LB/<100ppb EV. Group I in week 4 was inadvertently given a washout period at which time the steers consumed the amount of LB and EV given to group IV (control). Both feed and feces samples were extracted using difference solid phase extraction methods and quantified by HPLC-fluorescence for LB and EV. -

WO 2016/123419 Al 4 August 2016 (04.08.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2016/123419 Al 4 August 2016 (04.08.2016) P O P C T (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, C12Q 1/68 (2006.01) C07H 21/02 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, PCT/US2016/015503 KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, (22) International Filing Date: MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, 29 January 2016 (29.01 .2016) PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, (25) Filing Language: English TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 62/1 10,050 30 January 2015 (30.01.2015) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: PRESIDENT AND FELLOWS OF HAR¬ TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, VARD COLLEGE [US/US]; 17 Quincy Street, Cam DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, bridge, MA 02138 (US). -

The Role of BK Channels in Antiseizure Action of the CB1 Receptor Agonist ACEA in Maximal Electroshock and Pentylenetetrazole Models of Seizure in Mice
Iranian Journal of Pharmaceutical Research (2017), 16 (2): 640-647 Copyright © 2017 by School of Pharmacy Received: Aug. 2016 Shaheed Beheshti University of Medical Sciences and Health Services Accepted: Feb. 2017 Original Article The Role of BK Channels in Antiseizure Action of the CB1 Receptor Agonist ACEA in Maximal Electroshock and Pentylenetetrazole Models of Seizure in Mice Sina Asaadia, Mohammad Jahanbakhshia, Mahmoud Lotfiniab and Nima Naderia,c* aNeuroscience Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran. bSchool of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran. cDepartment of Pharmacology and Toxicology, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran. Abstract The anticonvulsant effect of cannabinoid compound has been shown in various models of seizure. On the other hand, there are controversial findings about the role of large conductance calcium-activated potassium (BK) channels in the pathogenesis of epilepsy. Also, there is no data regarding the effect of co-administration of cannabinoid type 1 (CB1) receptor agonists and BK channels antagonists in the acute models of seizure in mice. In this study, the effect of arachidonyl-2′-chloroethylamide (ACEA), a CB1 receptor agonist, and a BK channel antagonist, paxilline, either alone or in combination was investigated. Both pentylenetetrazole (PTZ) and maximal electroshock (MES) acute models of seizure were used to evaluate the protective effects of drugs. Mice were randomly selected in different groups: (i) control group; (ii) groups that received different doses of either paxilline or ACEA; and (iii) groups that received combinations of ACEA and paxillin at different doses. In MES model, prevention of hindlimb tonic extension (HLTE) was considered as protective effect. -

Fifth Annual Neuroscience, Behavior and Health Research Forum
FIFTH ANNUAL NEUROSCIENCE, BEHAVIOR AND HEALTH RESEARCH FORUM The University of Vermont Dudley H. Davis Center Livak Ballroom / Mansfield Room January 23 - 24, 2015 Platform Talks and Poster Abstracts Sponsored by: Society for Neuroscience Society for Neuroscience Vermont Chapter UVM Neuroscience, Behavior and Health Initiative UVM Neuroscience Graduate Program Neuroscience COBRE MBF Bioscience Med Associates / Catamount Research Platform Talk 1 Brain-derived neurotrophic factor: a novel regulator of cardiovascular function Benedek Erdos Department of Pharmacology, University of Vermont College of Medicine, Burlington, VT Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family and has a key role in regulating neuronal development and survival. In addition, increasing evidence indicates that neuronal activity-dependent production and release of BDNF provokes both short- term and long-term changes in synaptic function and that BDNF may also act as a neurotransmitter. The paraventricular nucleus of the hypothalamus (PVN) plays a central role in neural control of cardiovascular function, and BDNF synthesis in the PVN has been shown to increase in response to hypertensive stimuli including stress and hyperosmolarity. However, it is unclear whether BDNF, acting within the PVN, contributes to elevations in blood pressure. Using radiotelemetric blood pressure monitoring in Sprague-Dawley rats, we have established that 1) viral vector-mediated overexpression of BDNF in the PVN induces significant increases in mean arterial pressure; heart rate and indices of sympathetic activity; 2) acute microinjection of BDNF into the PVN elicits rapid elevations in blood pressure and heart rate; 3) both acute and long-term effects of BDNF are mediated at least in part by changes in the brain renin angiotensin system; 4) chronically elevated BDNF in the PVN upregulates catecholamine biosynthesizing enzymes in certain nuclei of the brainstem. -

Phytocannabinoids-And-Epilepsy.Pdf
Journal of Clinical Pharmacy and Therapeutics, 2014 doi: 10.1111/jcpt.12235 Review Article Phytocannabinoids and epilepsy R. G. dos Santos* PhD, J. E. C. Hallak*† MD PhD, J. P. Leite* MD PhD, A. W. Zuardi*† MD PhD and J. A. S. Crippa*† MD PhD *Department of Neuroscience and Behavior, Ribeir~ao Preto Medical School, University of S~ao Paulo, and †National Institute for Translational Medicine (INCT-TM), CNPq, Ribeir~ao Preto, Brazil Received 4 September 2014, Accepted 6 November 2014 Keywords: cannabidiol, cannabinoids, delta-9-tetrahydrocannabinol, epilepsy, seizure, treatment nabivarin (THCV), cannabidivarin (CBDV), delta-8-tetrahydrocan- SUMMARY nabinol (delta-8-THC), cannabinol (CBN) and especially CBD have – – What is known and objective: Antiepileptic drugs often produce anticonvulsant effects.13 17,23 29 Considering that currently avail- serious adverse effects, and many patients do not respond to able anti-epileptic drugs are not efficient for many patients and them properly. Phytocannabinoids produce anticonvulsant that these drugs often produce several side effects, there is a clear effects in preclinical and preliminary human studies, and appear need to explore other compounds with higher efficacy and lower to produce fewer adverse effects than available antiepileptic toxicity.23,24 Therefore, the present text presents a review of the drugs. The present review summarizes studies on the anticon- studies on the anticonvulsant effects of phytocannabinoids. vulsant properties of phytocannabinoids. Methods: Literature search using the PubMed database to METHODS identify studies on phytocannabinoids and epilepsy. Results and discussion: Preclinical studies suggest that phytoc- A literature search was performed in the PubMed database using annabinoids, especially cannabidiol and cannabidivarin, have the words ‘cannabis’, ‘marijuana’, ‘cannabinoids’, ‘tetrahydrocan- potent anticonvulsant effects which are mediated by the endoc- nabinol’, ‘THC’, ‘cannabidiol’, ‘CBD’, ‘cannabidivarin’, ‘CBDV’, annabinoid system.