Natural Modulators of Large-Conductance Calcium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tetrandrine Attenuates Left Ventricular Dysfunction in Rats with Myocardial Infarction
EXPERIMENTAL AND THERAPEUTIC MEDICINE 21: 119, 2021 Tetrandrine attenuates left ventricular dysfunction in rats with myocardial infarction YOUYANG WU, WEI ZHAO, FANHAO YE, SHIWEI HUANG, HAO CHEN, RUI ZHOU and WENBING JIANG Department of Cardiology, The Third Clinical Institute Affiliated to Wenzhou Medical University, Wenzhou, Zhejiang 325000, P.R. China Received March 16, 2020; Accepted September 16, 2020 DOI: 10.3892/etm.2020.9551 Abstract. The present study aimed to determine whether the levels of LVIDd and LVIDs were significantly higher; tetrandrine could attenuate left ventricular dysfunction and however, the levels of EF% and FS% were lower compared remodeling in rats with myocardial infarction. Sprague‑Dawley with those in the sham operation group, which was alleviated rats were randomly divided into six groups (n=5/group) as by tetrandrine. H&E results showed that tetrandrine allevi‑ follows: i) Healthy control group; ii) sham operation group; ated the pathological characteristics of myocardial infarction iii) myocardial infarction model group; iv) myocardial infarc‑ model rats. Furthermore, tetrandrine significantly inhibited tion + low‑dose tetrandrine group (10 mg/kg); v) myocardial myocardial cell apoptosis in rats with myocardial infarction. infarction + medium‑dose tetrandrine group (50 mg/kg); Tetrandrine significantly inhibited the levels of TG, TC and and vi) myocardial infarction + high‑dose tetrandrine group LDL and increased the levels of HDL in the arterial blood of (80 mg/kg). Left ventricular end‑diastolic diameter (LVIDd), rats with myocardial infarction. These findings revealed that left ventricular end‑systolic diameter (LVIDs), ejection frac‑ tetrandrine could attenuate left ventricular dysfunction in rats tion (EF%) and left ventricular fractional shortening rate (FS%) with myocardial infarction, which might be associated with were measured using ultrasonography. -

Specifications of Approved Drug Compound Library
Annexure-I : Specifications of Approved drug compound library The compounds should be structurally diverse, medicinally active, and cell permeable Compounds should have rich documentation with structure, Target, Activity and IC50 should be known Compounds which are supplied should have been validated by NMR and HPLC to ensure high purity Each compound should be supplied as 10mM solution in DMSO and at least 100µl of each compound should be supplied. Compounds should be supplied in screw capped vial arranged as 96 well plate format. -

De Novo BK Channel Variant Causes Epilepsy by Affecting Voltage Gating but Not Ca2+ Sensitivity
European Journal of Human Genetics (2018) 26:220–229 https://doi.org/10.1038/s41431-017-0073-3 ARTICLE De novo BK channel variant causes epilepsy by affecting voltage gating but not Ca2+ sensitivity 1 2 3,4 5 6 Xia Li ● Sibylle Poschmann ● Qiuyun Chen ● Walid Fazeli ● Nelly Jouayed Oundjian ● 7 8 9 9,10 Francesca M. Snoeijen-Schouwenaars ● Oliver Fricke ● Erik-Jan Kamsteeg ● Marjolein Willemsen ● Qing Kenneth Wang1,3,4 Received: 18 August 2017 / Revised: 6 November 2017 / Accepted: 23 November 2017 / Published online: 12 January 2018 © European Society of Human Genetics 2018 Abstract Epilepsy is one of the most common neurological diseases and it causes profound morbidity and mortality. We identified the first de novo variant in KCNMA1 (c.2984 A > G (p.(N995S)))—encoding the BK channel—that causes epilepsy, but not paroxysmal dyskinesia, in two independent families. The c.2984 A > G (p.(N995S)) variant markedly increased the macroscopic potassium current by increasing both the channel open probability and channel open dwell time. The c.2984 A > G (p.(N995S)) variant did not affect the calcium sensitivity of the channel. We also identified three other variants of fi > > > 1234567890 unknown signi cance (c.1554 G T (p.(K518N)), c.1967A C (p.(E656A)), and c.3476 A G (p.(N1159S))) in three separate patients with divergent epileptic phenotypes. However, these variants did not affect the BK potassium current, and are therefore unlikely to be disease-causing. These results demonstrate that BK channel variants can cause epilepsy without paroxysmal dyskinesia. The underlying molecular mechanism can be increased activation of the BK channel by increased sensitivity to the voltage-dependent activation without affecting the sensitivity to the calcium-dependent activation. -

Holocene Environmental Changes Disclosed from Anoxic Fjord Sediments by Biomarkers and Their Radiocarbon Content
GEOLOGICA ULTRAIECTINA Mededelingen van de Faculteit Geowetenschappen Universiteit Utrecht No. 227 Holocene environmental changes disclosed from anoxic fjord sediments by biomarkers and their radiocarbon content Rienk H. Smittenberg Holocene environmental changes disclosed from anoxic fjord sediments by biomarkers and their radiocarbon content Holocene milieuveranderingen gereconstrueerd uit anoxische fjord sedimenten middels biomarkers en hun radio-aktief koolstof gehalte (met een samenvatting in het Nederlands) Proefschrift ter verkrijging van de graad van doctor aan de Universiteit Utrecht op gezag van de Rector Magnificus, Prof. Dr. W.H. Gispen, ingevolge het besluit van het College voor Promoties in het openbaar te verdedigen op maandag 15 september 2003 des middags te 4.15 uur door Rienk Hajo Smittenberg geboren op 23 maart 1973 te Eck en Wiel Promotor: Prof. Dr. J.W. de Leeuw Department of Geochemistry Utrecht University Utrecht, The Netherlands Copromotores: Dr. Ir. J.S. Sinninghe Damsté Department of Geochemistry Utrecht University Utrecht, The Netherlands Dr. S. Schouten Department of Marine Biogeochemistry and Toxicology Royal Netherlands Institute of Sea Research Texel, The Netherlands The research described in this thesis was carried out at the Department of Biogeochemistry and Toxicology of the Royal Netherlands Institute of Sea Research, P.O. Box 59, 1790 AB Den Burg, The Netherlands. The investigations were supported by the Research Council for Earth and Life Science (ALW) with the financial support from the Netherlands -
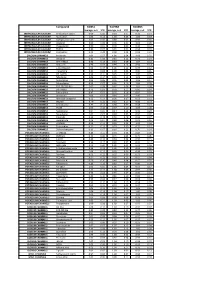
Biomol Average and SD Table S1.Xlsx
Compound GliNS1 G179NS G166NS average, n=5 STD average, n=3 STD average, n=3 STD INTRACELLULAR CALCIUM Antibiotic A-23187 0.00 0.00 0.10 0.07 0.15 0.14 INTRACELLULAR CALCIUM Ryanodine 1.04 0.14 1.03 0.03 1.03 0.03 INTRACELLULAR CALCIUM Cyclopiazonic acid 1.01 0.06 0.88 0.05 0.92 0.06 INTRACELLULAR CALCIUM Gingerol 1.00 0.06 0.91 0.01 1.01 0.06 INTRACELLULAR CALCIUM Thapsigargin 0.00 0.01 0.00 0.00 0.10 0.12 INTRACELLULAR CALCIUM TMB-8 0.89 0.07 0.91 0.05 0.94 0.03 INTRACELLULAR CALCIUM Dantrolene 0.91 0.08 0.98 0.05 0.94 0.01 CALCIUM CHANNELS Amiloride 1.01 0.07 1.01 0.04 1.03 0.05 CALCIUM CHANNELS Benzamil 0.83 0.08 0.83 0.12 0.96 0.04 CALCIUM CHANNELS BAY K-8644 0.93 0.13 0.93 0.09 1.07 0.14 CALCIUM CHANNELS Diltiazem 0.96 0.07 0.99 0.12 0.94 0.14 CALCIUM CHANNELS L-cis-Diltiazem 0.91 0.17 1.01 0.12 0.95 0.12 CALCIUM CHANNELS Flunarizine 0.85 0.08 1.00 0.06 0.85 0.05 CALCIUM CHANNELS FPL-64176 0.99 0.11 0.95 0.07 1.05 0.05 CALCIUM CHANNELS Nifedipine 1.06 0.17 0.95 0.12 1.03 0.09 CALCIUM CHANNELS Nimodipine 1.05 0.06 0.95 0.03 1.06 0.17 CALCIUM CHANNELS Nitrendipine 0.99 0.07 0.96 0.10 1.04 0.09 CALCIUM CHANNELS SDZ-202791 R(-) 1.01 0.08 0.92 0.06 1.01 0.08 CALCIUM CHANNELS SKF-96365 0.73 0.05 0.70 0.11 0.69 0.04 CALCIUM CHANNELS Tetrandrine 0.47 0.07 0.76 0.16 0.87 0.20 CALCIUM CHANNELS Verapamil 1.01 0.02 0.89 0.07 1.06 0.20 CALCIUM CHANNELS Methoxy Verapamil 0.93 0.14 0.96 0.07 0.93 0.13 CALCIUM CHANNELS Bepridil 0.70 0.16 0.92 0.15 0.84 0.14 CALCIUM CHANNELS Amiodarone 0.32 0.12 0.58 0.07 0.48 0.23 CALCIUM CHANNELS YS035 1.00 0.16 -

Application of Biomarker Compounds As Tracers for Sources and Fates of Natural and Anthropogenic Organic Matter in Tile Environment
AN ABSTRACT OF THE DISSERTATION OF Daniel R. Oros for the degree of Doctor of Philosophy in Environmental Sciences presented on September 24. 1999. Title: Application of Biomarker Compoundsas Tracers for Sources and Fates of Natural and Anthropogenic Organic Matter in the Environment. Redacted for Privacy Abstract approved: Bernd R.T. Simoneit Determination of the source and fate of natural (higher plant lipids, marine lipids, etc.) and anthropogenically (e.g., petroleum, coal emissions) derived hydrocarbons and oxygenated compounds in the environment was accomplished using gas chromatography (GC) and gas chromatography-mass spectrometry (GC- MS) to characterize or identify molecular biomarkers to be utilized as tracers. The distributions and abundances of biomarkers such as straight chain homologous series (e.g., n-alkanes, n-alkanoic acids, n-alkan-2-ones, n-alkanols, etc.) and cyclic terpenoid compounds (e.g., sesquiterpenoids, diterpenoids, steroids, triterpenoids) were identified in epicuticular waxes from conifers of western North America (natural emissions). These biomarkers and their thermal alteration derivativeswere also identified in smoke emissions from known vegetation sources (e.g., conifers, deciduous trees and grasses) and were then applied as tracers in soils, soils that contained wildfire residues and soillriver mud washout after wildfire burning. Where possible, the reaction pathways of transformation from the parentprecursor compounds to intermediate and final alteration products were determined from GC- MS data. In addition, molecular tracer analysis was applied to air, water and sediment samples collected from a lacustrine setting (Crater Lake, OR) in order to determine the identities, levels and fates of anthropogenic (i.e., petroleum hydrocarbon contamination from boating and related activities) hydrocarbons ina pristine organic matter sink. -

Emerging Roles for Multifunctional Ion Channel Auxiliary Subunits in Cancer T ⁎ Alexander S
Cell Calcium 80 (2019) 125–140 Contents lists available at ScienceDirect Cell Calcium journal homepage: www.elsevier.com/locate/ceca Emerging roles for multifunctional ion channel auxiliary subunits in cancer T ⁎ Alexander S. Hawortha,b, William J. Brackenburya,b, a Department of Biology, University of York, Heslington, York, YO10 5DD, UK b York Biomedical Research Institute, University of York, Heslington, York, YO10 5DD, UK ARTICLE INFO ABSTRACT Keywords: Several superfamilies of plasma membrane channels which regulate transmembrane ion flux have also been Auxiliary subunit shown to regulate a multitude of cellular processes, including proliferation and migration. Ion channels are Cancer typically multimeric complexes consisting of conducting subunits and auxiliary, non-conducting subunits. Calcium channel Auxiliary subunits modulate the function of conducting subunits and have putative non-conducting roles, further Chloride channel expanding the repertoire of cellular processes governed by ion channel complexes to processes such as trans- Potassium channel cellular adhesion and gene transcription. Given this expansive influence of ion channels on cellular behaviour it Sodium channel is perhaps no surprise that aberrant ion channel expression is a common occurrence in cancer. This review will − focus on the conducting and non-conducting roles of the auxiliary subunits of various Ca2+,K+,Na+ and Cl channels and the burgeoning evidence linking such auxiliary subunits to cancer. Several subunits are upregu- lated (e.g. Cavβ,Cavγ) and downregulated (e.g. Kvβ) in cancer, while other subunits have been functionally implicated as oncogenes (e.g. Navβ1,Cavα2δ1) and tumour suppressor genes (e.g. CLCA2, KCNE2, BKγ1) based on in vivo studies. The strengthening link between ion channel auxiliary subunits and cancer has exposed these subunits as potential biomarkers and therapeutic targets. -

Chemical Synthesis, Proper Folding, Nav Channel Selectivity Profile And
toxins Article Chemical Synthesis, Proper Folding, Nav Channel Selectivity Profile and Analgesic Properties of the Spider Peptide Phlotoxin 1 1, 2,3, 4,5, 1 Sébastien Nicolas y, Claude Zoukimian y, Frank Bosmans y,Jérôme Montnach , Sylvie Diochot 6, Eva Cuypers 5, Stephan De Waard 1,Rémy Béroud 2, Dietrich Mebs 7 , David Craik 8, Didier Boturyn 3 , Michel Lazdunski 6, Jan Tytgat 5 and Michel De Waard 1,2,* 1 Institut du Thorax, Inserm UMR 1087/CNRS UMR 6291, LabEx “Ion Channels, Science & Therapeutics”, F-44007 Nantes, France; [email protected] (S.N.); [email protected] (J.M.); [email protected] (S.D.W.) 2 Smartox Biotechnology, 6 rue des Platanes, F-38120 Saint-Egrève, France; [email protected] (C.Z.); [email protected] (R.B.) 3 Department of Molecular Chemistry, Univ. Grenoble Alpes, CNRS, 570 rue de la chimie, CS 40700, 38000 Grenoble, France; [email protected] 4 Faculty of Medicine and Health Sciences, Department of Basic and Applied Medical Sciences, 9000 Gent, Belgium; [email protected] 5 Toxicology and Pharmacology, University of Leuven, Campus Gasthuisberg, P.O. Box 922, Herestraat 49, 3000 Leuven, Belgium; [email protected] (E.C.); [email protected] (J.T.) 6 Université Côte d’Azur, CNRS UMR7275, Institut de Pharmacologie Moléculaire et Cellulaire, 660 route des lucioles, 6560 Valbonne, France; [email protected] (S.D.); [email protected] (M.L.) 7 Institute of Legal Medicine, University of Frankfurt, Kennedyallee 104, 60488 Frankfurt, Germany; [email protected] 8 Institute for Molecular Bioscience, University of Queensland, Brisbane 4072, Australia; [email protected] * Correspondence: [email protected]; Tel.: +33-228-080-076 Contributed equally to this work. -

Iberiotoxin-Induced Block of Kca Channels Induces Dihydropyridine
JPET Fast Forward. Published on January 24, 2003 as DOI: 10.1124/jpet.102.046102 JPET FastThis articleForward. has not Published been copyedited on and January formatted. 24,The final2003 version as DOI:10.1124/jpet.102.046102 may differ from this version. JPET/2002/46102 Iberiotoxin-induced Block of KCa Channels Induces Dihydropyridine Sensitivity of ACh Release from Mammalian Motor Nerve Terminals Downloaded from Michael T. Flink and William D. Atchison Department of Pharmacology & Toxicology jpet.aspetjournals.org Michigan State University E. Lansing, MI 48824 Address all correspondence including reprint requests to: at ASPET Journals on September 23, 2021 Dr. Bill Atchison Dept. Pharmacology & Toxicology Michigan State University B-331 Life Sciences Bldg. East Lansing, MI 48824-1317 Phone: (517) 353-4947 Fax: (517) 432-1341 Email: [email protected] 1 Copyright 2003 by the American Society for Pharmacology and Experimental Therapeutics. JPET Fast Forward. Published on January 24, 2003 as DOI: 10.1124/jpet.102.046102 This article has not been copyedited and formatted. The final version may differ from this version. JPET/2002/46102 Running Title: KCa Channel Block and DHP-sensitivity of ACh Release at NMJ (59 spaces) Document Statistics: Text Pages: 15 Tables: 1 Figures: 5 References: 42 Downloaded from Abstract: 249 words Introduction: 760 words Discussion: 1241 jpet.aspetjournals.org at ASPET Journals on September 23, 2021 LIST OF ABBREVIATIONS: ACh, acetylcholine; BSA, bovine serum albumin; Cav, voltage-activated calcium channel; DAP, 3,4 diaminopyridine; DHP, dihydropyridine;EPP, end-plate potential; HEPES, N-2- hydroxyethylpiperazine-N-2-ethanesulfonic acid ; KCa, calcium-activated potassium; MEPP, miniature end-plate potential 2 JPET Fast Forward. -

Expression Profiling of Ion Channel Genes Predicts Clinical Outcome in Breast Cancer
UCSF UC San Francisco Previously Published Works Title Expression profiling of ion channel genes predicts clinical outcome in breast cancer Permalink https://escholarship.org/uc/item/1zq9j4nw Journal Molecular Cancer, 12(1) ISSN 1476-4598 Authors Ko, Jae-Hong Ko, Eun A Gu, Wanjun et al. Publication Date 2013-09-22 DOI http://dx.doi.org/10.1186/1476-4598-12-106 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Ko et al. Molecular Cancer 2013, 12:106 http://www.molecular-cancer.com/content/12/1/106 RESEARCH Open Access Expression profiling of ion channel genes predicts clinical outcome in breast cancer Jae-Hong Ko1, Eun A Ko2, Wanjun Gu3, Inja Lim1, Hyoweon Bang1* and Tong Zhou4,5* Abstract Background: Ion channels play a critical role in a wide variety of biological processes, including the development of human cancer. However, the overall impact of ion channels on tumorigenicity in breast cancer remains controversial. Methods: We conduct microarray meta-analysis on 280 ion channel genes. We identify candidate ion channels that are implicated in breast cancer based on gene expression profiling. We test the relationship between the expression of ion channel genes and p53 mutation status, ER status, and histological tumor grade in the discovery cohort. A molecular signature consisting of ion channel genes (IC30) is identified by Spearman’s rank correlation test conducted between tumor grade and gene expression. A risk scoring system is developed based on IC30. We test the prognostic power of IC30 in the discovery and seven validation cohorts by both Cox proportional hazard regression and log-rank test. -

Potassium Channels in Peripheral Pain Pathways: Expression, Function and Therapeutic Potential
Send Orders for Reprints to [email protected] Current Neuropharmacology, 2013, 11, 621-640 621 Potassium Channels in Peripheral Pain Pathways: Expression, Function and Therapeutic Potential Xiaona Du1,* and Nikita Gamper1,2,* 1Department of Pharmacology, Hebei Medical University, Shijiazhuang, China; 2Faculty of Biological Sciences, University of Leeds, Leeds, UK Abstract: Electrical excitation of peripheral somatosensory nerves is a first step in generation of most pain signals in mammalian nervous system. Such excitation is controlled by an intricate set of ion channels that are coordinated to produce a degree of excitation that is proportional to the strength of the external stimulation. However, in many disease states this coordination is disrupted resulting in deregulated peripheral excitability which, in turn, may underpin pathological pain states (i.e. migraine, neuralgia, neuropathic and inflammatory pains). One of the major groups of ion channels that are essential for controlling neuronal excitability is potassium channel family and, hereby, the focus of this review is on the K+ channels in peripheral pain pathways. The aim of the review is threefold. First, we will discuss current evidence for the expression and functional role of various K+ channels in peripheral nociceptive fibres. Second, we will consider a hypothesis suggesting that reduced functional activity of K+ channels within peripheral nociceptive pathways is a general feature of many types of pain. Third, we will evaluate the perspectives of pharmacological enhancement of K+ channels in nociceptive pathways as a strategy for new analgesic drug design. + + Keywords: K channel/ M channel/ two-pore K channel/ KATP channel/ Dorsal root ganglion/ Pain/ Nociception. INTRODUCTION TRPV1 [4] while strong mechanical stimulation activates mechanosensitive channels which can be Piezo2 [5]. -

Slow Inactivation in Voltage Gated Potassium Channels Is Insensitive to the Binding of Pore Occluding Peptide Toxins
Biophysical Journal Volume 89 August 2005 1009–1019 1009 Slow Inactivation in Voltage Gated Potassium Channels Is Insensitive to the Binding of Pore Occluding Peptide Toxins Carolina Oliva, Vivian Gonza´lez, and David Naranjo Centro de Neurociencias de Valparaı´so, Facultad de Ciencias, Universidad de Valparaı´so, Valparaı´so, Chile ABSTRACT Voltage gated potassium channels open and inactivate in response to changes of the voltage across the membrane. After removal of the fast N-type inactivation, voltage gated Shaker K-channels (Shaker-IR) are still able to inactivate through a poorly understood closure of the ion conduction pore. This, usually slower, inactivation shares with binding of pore occluding peptide toxin two important features: i), both are sensitive to the occupancy of the pore by permeant ions or tetraethylammonium, and ii), both are critically affected by point mutations in the external vestibule. Thus, mutual interference between these two processes is expected. To explore the extent of the conformational change involved in Shaker slow inactivation, we estimated the energetic impact of such interference. We used kÿconotoxin-PVIIA (kÿPVIIA) and charybdotoxin (CTX) peptides that occlude the pore of Shaker K-channels with a simple 1:1 stoichiometry and with kinetics 100-fold faster than that of slow inactivation. Because inactivation appears functionally different between outside-out patches and whole oocytes, we also compared the toxin effect on inactivation with these two techniques. Surprisingly, the rate of macroscopic inactivation and the rate of recovery, regardless of the technique used, were toxin insensitive. We also found that the fraction of inactivated channels at equilibrium remained unchanged at saturating kÿPVIIA.