Patterns in Evolution in Characters That Define Iris Subgenera And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Taxonomic Status of Gladiolus Illyricus (Iridaceae) in Britain
The Taxonomic Status of Gladiolus illyricus (Iridaceae) in Britain Aeron Buchanan Supervisor: Fred Rumsey, Natural History Museum, London A thesis submitted in partial fulfilment of the requirements for the degree of Master of Science of Imperial College, London Abstract First noticed officially in Britain in 1855, Gladiolus illyricus (Koch) presents an interesting taxonomic and biogeographical challenge: whether or not this isolated northern population should be recognized as a separate sub-species. Fundamental conservation issues rest on the outcome. Here, the investigation into the relationship of the G. illyricus plants of the New Forest, Hampshire, to Gladiolus species across Europe, northern Africa and the middle east is initiated. Two chloroplast regions, one in trnL–trnF and the other across psbA–trnH have been sequenced for 42 speci- mens of G. illyricus, G. communis, G. italicus, G. atroviolaceus, G. triphyllos and G. anatolicus. Phylogenetic and biogeographical treatments support the notion of an east–west genetic gradation along the Mediterranean. Iberia particularly appears as a zone of high hybridization potential and the source of the New Forest population. Alignment with sequences obtained from GenBank give strong support to the classic taxonomy of Gladiolus being monophyletic in its sub-family, Ixioideae. Comments on these chloroplast regions for barcoding are also given. In conclusion, the genetic localization of Britain’s G. illyricus population as an extremity haplotype suggests that it could well deserve sub-species status. Contents 1 Introduction 2 2 Background 4 3 Materials and Methods 8 4 Results and Discussion 15 5 Conclusions 26 Appendices 28 References 56 1. Introduction G. illyricus in Britain Figure 1: G. -
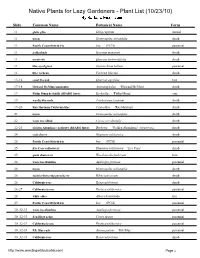
Native Plants for Lazy Gardeners - Plant List (10/23/10)
Native Plants for Lazy Gardeners - Plant List (10/23/10) Slide Common Name Botanical Name Form 11 globe gilia Gilia capitata annual 11 toyon Heteromeles arbutifolia shrub 11 Pacific Coast Hybrid iris Iris (PCH) perennial 11 goldenbush Isocoma menziesii shrub 11 scrub oak Quercus berberidifolia shrub 11 blue-eyed grass Sisyrinchium bellum perennial 11 lilac verbena Verbena lilacina shrub 13-16 coast live oak Quercus agrifolia tree 17-18 Howard McMinn man anita Arctostaphylos 'Howard McMinn' shrub 19 Philip Mun keckiella (RSABG Intro) Keckiella 'Philip Munz' ine 19 woolly bluecurls Trichostema lanatum shrub 19-20 Ray Hartman California lilac Ceanothus 'Ray Hartman' shrub 21 toyon Heteromeles arbutifolia shrub 22 western redbud Cercis occidentalis shrub 22-23 Golden Abundance barberry (RSABG Intro) Berberis 'Golden Abundance' (MAHONIA) shrub 2, coffeeberry Rhamnus californica shrub 25 Pacific Coast Hybrid iris Iris (PCH) perennial 25 Eve Case coffeeberry Rhamnus californica '. e Case' shrub 25 giant chain fern Woodwardia fimbriata fern 26 western columbine Aquilegia formosa perennial 26 toyon Heteromeles arbutifolia shrub 26 fuchsia-flowering gooseberry Ribes speciosum shrub 26 California rose Rosa californica shrub 26-27 California fescue Festuca californica perennial 28 white alder Alnus rhombifolia tree 29 Pacific Coast Hybrid iris Iris (PCH) perennial 30 032-33 western columbine Aquilegia formosa perennial 30 032-33 San Diego sedge Carex spissa perennial 30 032-33 California fescue Festuca californica perennial 30 032-33 Elk Blue rush Juncus patens '.l1 2lue' perennial 30 032-33 California rose Rosa californica shrub http://www weedingwildsuburbia com/ Page 1 30 032-3, toyon Heteromeles arbutifolia shrub 30 032-3, fuchsia-flowering gooseberry Ribes speciosum shrub 30 032-3, Claremont pink-flowering currant (RSA Intro) Ribes sanguineum ar. -

A HANDBOOK of GARDEN IRISES by W
A HANDBOOK OF GARDEN IRISES By W. R. DYKES, M.A., L.-ès-L. SECRETARY OF THE ROYAL HORTICULTURAL SOCIETY. AUTHOR OF "THE GENUS IRIS," ETC. CONTENTS. PAGE PREFACE 3 1 THE PARTS OF THE IRTS FLOWER AND PLANT 4 2 THE VARIOUS SECTIONS OF THE GENUS AND 5 THEIR DISTRIBUTION 3 THE GEOGRAPHICAL DISTRIBUTION OF THE VARIOUS 10 SECTIONS AND SPECIES AND THEIR RELATIVE AGES 4 THE NEPALENSIS SECTION 13 5 THE GYNANDRIRIS SECTION 15 6 THE RETICULATA SECTION 16 7 THE JUNO SECTION 23 8 THE XIPHIUM SECTION 33 9 THE EVANSIA SECTION 40 10 THE PARDANTHOPSIS SECTION 45 11 THE APOGON SECTION 46 — THE SIBIRICA SUBSECTION 47 — THE SPURIA SUBSECTION 53 — THE CALIFORNIAN SUBSECTION 59 — THE LONGIPETALA SUBSECTION 64 — THE HEXAGONA SUBSECTION 67 — MISCELLANEOUS BEARDLESS IRISES 69 12 THE ONCOCYCLUS SECTION 77 I. Polyhymnia, a Regeliocydus hybrid. 13 THE REGELIA SECTION 83 (I. Korolkowi x I. susianna). 14 THE PSEUDOREGELIA SECTION 88 15 THE POGONIRIS SECTION 90 16 GARDEN BEARDED IRISES 108 17 A NOTE ON CULTIVATION, ON RAISING 114 SEEDLINGS AND ON DISEASES 18 A TABLE OF TIMES OF PLANTING AND FLOWERING 116 19 A LIST OF SYNONYMS SOMETIMES USED IN 121 GARDENS This edition is copyright © The Goup for Beardless Irises 2009 - All Rights Reserved It may be distributed for educational purposes in this format as long as no fee (or other consideration) is involved. www.beardlessiris.org PREFACE TO THIS DIGITAL EDITION William Rickatson Dykes (1877-1925) had the advantage of growing irises for many years before writing about them. This Handbook published in 1924 represents the accumulation of a lifetime’s knowledge. -

Philip J. Savage, Jr
ISSUE 73 M4SNOU4 Philip J. Savage, Jr. May 8, 1917 — October 13, 2002 Phil Savnge, /r. , an icon m the Magnolia worhl, passed au ay last October of conr plica- lions from the West Nile virus. Phil, a renotvrred magnolin Irybcidrzer, specialized in breeding mngnolias that u&cre cold Irardy. Becnuse of this, nragnolia entirusiasts living in colder climalcs now have morry rrrorc choices thnn did Dennis Ledvh rn u&hen he bought Iris lrouse irr Green Bay, Wiscorrsin in thc Intr typos Plril hns left an errduring legaclt n&itlr tire mnny /Inc, cold-lurrdy hybrids Ire bred. Following arc set&eral rwni niscclrces fronr irrdividuals who toere deqviy in/luencerl by Phil mrd Iris u&ork u&ith mngrrolins. DENNIs LEDVINA WIUTES. Back in the late yos the landscaping around my new house consisted of three magnolias: two M. x sorrlarrgeana, and a M. x loebneri 'MerrilL' At the time, these were the only magnolias gener- ally available at local nurseries. As I watched these magnolias bloom each spring, I became more intrigued with their beautiful Bowers and began driv- ing around Green Bay to observe and admire some of the established trees. My admiration for the genus contin- ued to grow each year as I began col- lecting more information about these magnificent plants. One summer I was in the Detroit area and I decided to call this magnolia expert, Phil Savage, that I had read so much about. I can vividly remember calling Phil from a telephone booth on Telegraph Road and finding mysell, an unknown amateur, talking to a magnolia expert who from the first made me feel like a lifelong friend. -

Recovery Plan for the Grevillea Beadleana
Approved NSW & National Recovery Plan Recovery Plan for the Grevillea beadleana July 2004 Department of Environment and Conservation (NSW) © NSW Department of Environment and Conservation, 2004. This work is copyright. However, material presented in this plan may be copied for personal use or published for educational purposes, providing that any extracts are fully acknowledged. Apart from this and any other use as permitted under the Copyright Act 1968, no part may be reproduced without prior written permission from the Department of Environment and Conservation. NSW Department of Environment and Conservation 43 Bridge Street (PO Box 1967) Hurstville NSW 2220 Tel: 02 9585 6444 www.nationalparks.nsw.gov.au Requests for information or comments regarding the recovery program for the Grevillea beadleana are best directed to: The Grevillea beadleana Recovery Coordinator Threatened Species Unit, North East Branch NSW Department of Environment and Conservation Locked Bag 914 Coffs Harbour NSW 2450 Tel 02 6651 5946 Cover illustration: Tina Woolfe This plan should be cited as follows: NSW Department of Environment and Conservation (2004), Approved Recovery Plan for the Grevillea beadleana, NSW Department of Environment and Conservation, Hurstville. ISBN 174122 135 8 Approved Recovery Plan Grevillea beadleana Recovery Plan for the Grevillea beadleana Foreword The New South Wales Government established a new environment agency on 24 September 2003, the Department of Environment and Conservation, which incorporates the New South Wales National Parks and Wildlife Service. Responsibility for the preparation of Recovery Plans now rests with this new department. This document constitutes the formal New South Wales State Recovery Plan for the Grevillea beadleana and considers the conservation requirements of the species across its known range. -

Radiation in the Cape Flora and the Phylogeny of Peacock Irises Moraea
ARTICLE IN PRESS MOLECULAR PHYLOGENETICS AND EVOLUTION Molecular Phylogenetics and Evolution xxx (2002) xxx–xxx www.academicpress.com Radiation in the Cape flora and the phylogeny of peacock irises Moraea (Iridaceae) based on four plastid DNA regions Peter Goldblatt,a Vincent Savolainen,b,* Obie Porteous,b Ivan Sostaric,b Martyn Powell,b Gail Reeves,c John C. Manning,c Timothy G. Barraclough,d and Mark W. Chaseb a Missouri Botanical Garden, P.O. Box 299, St. Louis, Missouri 63166, USA b Molecular Systematics Section, Jodrell Laboratory, Royal Botanic Gardens, Kew, Richmond TW9 3DS, UK c National Botanical Institute, Kirstenbosch, Private Bag X7, Cape Town, South Africa d Department of Biology and NERC Centre for Population Biology, Imperial College, Silwood Park, Ascot, Berkshire SL5 7PY, UK Received 2 February 2002; received in revised form 22 April 2002 Abstract Phylogenetic analyses of four plastid DNA regions, the rbcL exon, trnL intron, trnL–trnF intergenic spacer, and rps16 intron from each of 73 species in the African genus Moraea (Iridaceae: Irideae) including accessions of all major species clusters in the genus, show Moraea to be paraphyletic when Barnardiella, Galaxia, Hexaglottis, Homeria (all southern African), and Gynandriris (Eurasian as well) were recognized as separate genera. There are several small, isolated species clusters at the basal nodes of the tree that are all restricted to the winter-rainfall zone of southern Africa (the Greater Cape floral kingdom) and a few, highly derived, large species groups that have radiated extensively within the winter-rainfall zone. Mapping of floral traits shows that an Iris-type flower is ancestral in Moraea. -

May 15, 2016 Passing Peony and Iris Plants on from Generation to Generation Annette Meyer Heisdorffer Daviess County Extension Agent for Horticulture
May 15, 2016 Passing Peony and Iris Plants on from Generation to Generation Annette Meyer Heisdorffer Daviess County Extension Agent for Horticulture After lunch on Mother’s Day, my mom and I surveyed her garden, especially the peonies. We both agreed that I needed to propagate her peonies and plant them in my garden. These are special, because I remember them growing in my grandmother’s garden. Peonies are commonly passed down from generation to generation. My goal is to someday share them with my twins. Our discussion included the irises, which are another heritage plant. Both plants are blooming beautifully in May and are spectacular in the garden. Information about these two plants will be provided in this article. Peony (Paeonia officinalis, Paeonia lactiflora, and hybrids) is a herbaceous perennial, which means at the end of the growing season it will die back to the ground. However, the plant returns year after year. Peonies grow best in full sun and well-drained soil. There are tree peonies (Paeonia suffruticosa) which have a woody stem, but those are not as common and require different growing conditions. The tree peony will not be discussed here. According to Dr. Rick Durham, Extension Specialist for Consumer Horticulture, peonies can be found in landscapes across Kentucky. Peonies have a long life span and are commonly grown in the garden. When planting the root, make sure it is not too deep. The eyes or bud should be just below the surface of the soil. If it is planted too deeply, the plants won't bloom. -

SIGNA: Species Iris Group of North America 31Th Species Seed Exchange
SIGNA: Species Iris Group of North America 1997 o 31th Species Seed Exchange Greetings: Orders will be filled in the order received. Return immediately for the best selection. Our first shipment of seeds will begin January 10. Orders received after that date will be filled as time permits. No orders will be filled if received after March 1, 1998. After each item in the seed list you will find a number estimating the total number of seeds available. Donations with fewer than 100 seeds will most likely be sold out early. Be sure to check substitutes when ordering any of these seeds. They will not be used as substitutes. Seeds in short supply may be packed with as few as 4 seeds. If you want items with more seeds per packet, order items in greater supply. Please note the following abreviations used in the seedlist: H P means Hand Pollinated, coli. means Wild Collected, and ex. indicates that the plants that seeds were collected from were originally from another source (which may be a person, another seed exchange, or a wild location) which immediately follows the abbreviation. The alphabetical groups (A, B, C, etc.) used in the seed list follow the outline provided in the SIGNA Species Iris Study Manual'publlshed in 1972, e.g. sub-section Pogoniris, series Pumilae is under A, sub-section Pogoniris, series Intermedeae in under B and so on. The Study Manual , The Iris by Brian Mathew, and Iris of China by James Waddick and Zhao Yu-tang are used as references when verifying names. -

Seed Dispersal by Ants in Jarrah Forest Restorations of Western Australia
Seed Dispersal by Ants in Jarrah Forest Restorations of Western Australia Troy L. Wanless Introduction The jarrah forests in Western Australia cover approximately 1.75 million ha in the southwestern corner of the state (Figure 1). Jarrah, otherwise known as Eucalyptus marginata, is only one species among many that inhabit a region with considerable physical and biological diversity. 1200 species of plants, 29 mammalian species, 45 reptile species, 17 frog species, 4 fish species and 150 bird species live in this system which also has highly adverse conditions for survival. These may include infertile, often salt-laden soils, drought, and the occasional wildfire (Western Australia Forest Alliance, 2003). Considerable deposits of bauxite, which is the primary material involved in the production of aluminum, are scattered throughout the region. Since 1963, Alcoa of Australia Ltd. has cleared these jarrah forests to make way for mining of this ore. It is estimated that these deposits cover 7-8% of the forested area although only 3-4% will ever be mined due to environmental and economic constraints (Majer, 1989). These mined areas create a scattering of patches throughout the forest that are essentially stripped of any kind of biodiversity that was once there (Figure 2). Restoration efforts on these previously mined patches focus on many aspects of the jarrah forest ecosystem. Specifically interesting is the role that ants play in seed dispersal. This paper will focus on the topic of seed dispersal by ants in the northern jarrah forests of Western Australia while paying particular attention to myrmecochory. Figure 1. Location of jarrah forest in Australia Figure 2. -

Large-Flowered Trilliums Coming up in Spring in Our Childhoo
Large-flowered Trillium or Common Trillium (Trillium grandiflorum) Large-flowered Trilliums Coming Up in Spring In our childhood the woods in late May were full of hundreds of large, waxy white flowers that covered the forest floor. Grandma in her German diary called these trilliums “wild lilies” and also commented that on May14th and 22nd in 1927 and again on May 22nd in 1940 these flowers were numerous and in full bloom. (Freckman, 1994) gave the earliest blooming dates as April 24th. Large-flowered Trillium Bud Opening Today, with more houses and lawns in the country, and fewer undisturbed woodlots those visions of white are less frequently seen. In addition, the White-tailed Deer population has steadily increased and their appetite for tasty trilliums has served to eliminate many stands of this beautiful plant. This is especially troublesome because once the leaves and flowers are plucked, the plant is likely to die. It can no longer produce food to send down to the roots so that it can come back another year. It still grows quite abundantly on the sloping bank along Billings Avenue in the Town of Medford, Wisconsin. Some sources say that deer do not like to graze on steep banks or inclines so that may be why those plants have escaped. We have a patch of trilliums that has grown larger each year in our lawn. Recently we have had to protect these trilliums from the deer that could wipe out the entire patch in a single night. Sometimes seeds from those plants, possibly transported by ants, have grown into new plants in the front lawn. -

Scanned Document
•••••OCTOBER · 19 7 4- Number 14 THE SPECIES IRIS STUDY GROUP OF THE AMERICAN IRIS SOCIETY Jean Witt, of Seattle, is the Director of the AIS Species Seed Exchange. he also is an expert at doing ink-line botanic illustrations. Her seed exchange list for 1974 will be exten ive - but it will NOT offer eeds of the pecies which she has drawn for this cover of SIGNA. Seeds of Iris afghanica, of tpe Regelia Section, are not yet available - because Iris afghanico is a newly discovered and newly described species. More details are on page 367. THE SPECIES IRIS STUDY GROUP of_ TH E AMERICAN IRIS SOCIETY OFFICERS OF THE SOCIETY Chairman- - - - - - - Roy Davidson- - - 911 Western Avenue,,_ Number 200 Seattle, Washington !:18104 phone 206-746- 2156 Secretary-Treasurer - - - Homer Metcalf - - Montana State Universi~i College of Agriculture BoLeman Montana 597 5 phone 46 6-586-5624 Librarian - - - - - - Jerry Flintoff- 5608 North 18th Street Tacoma, Washi:1gton 98406 Seed Exchange Director Jean Witt - 16516 25th, N.E. Seattle, Washington 98155 Species Robins Director- Lorena Reid 17225 McKenzie Highwa'i, Route 2 Springfield, Oregon 97477 Editor of SIGNA - - - Bill Gunther 740 Crest Road Del Ma.c, California 92014 phone , 14-755- 2798 Editor of Study Manual Roy Davidson- - 911 Western Avenue,,_ Number 200 Seattle, Washington !:18104 • • • • • • • • • • • SIGNA - - - Number 14 OCTOBER 1974 TABLE OF CONTENTS Cover--lris afghanica · Jean Witt - · · · 353 Notes on SIGNA 13 · - Roy Davidson - - · 355 It is a Gift! - - - - - Bill Gunther · · · 356 The Genus Iris: a review - - - - - - - P.J. Chittenden - - 357 Spuria Species as Garden Plants - E. -

Native Plants Sixth Edition Sixth Edition AUSTRALIAN Native Plants Cultivation, Use in Landscaping and Propagation
AUSTRALIAN NATIVE PLANTS SIXTH EDITION SIXTH EDITION AUSTRALIAN NATIVE PLANTS Cultivation, Use in Landscaping and Propagation John W. Wrigley Murray Fagg Sixth Edition published in Australia in 2013 by ACKNOWLEDGEMENTS Reed New Holland an imprint of New Holland Publishers (Australia) Pty Ltd Sydney • Auckland • London • Cape Town Many people have helped us since 1977 when we began writing the first edition of Garfield House 86–88 Edgware Road London W2 2EA United Kingdom Australian Native Plants. Some of these folk have regrettably passed on, others have moved 1/66 Gibbes Street Chatswood NSW 2067 Australia to different areas. We endeavour here to acknowledge their assistance, without which the 218 Lake Road Northcote Auckland New Zealand Wembley Square First Floor Solan Road Gardens Cape Town 8001 South Africa various editions of this book would not have been as useful to so many gardeners and lovers of Australian plants. www.newhollandpublishers.com To the following people, our sincere thanks: Steve Adams, Ralph Bailey, Natalie Barnett, www.newholland.com.au Tony Bean, Lloyd Bird, John Birks, Mr and Mrs Blacklock, Don Blaxell, Jim Bourner, John Copyright © 2013 in text: John Wrigley Briggs, Colin Broadfoot, Dot Brown, the late George Brown, Ray Brown, Leslie Conway, Copyright © 2013 in map: Ian Faulkner Copyright © 2013 in photographs and illustrations: Murray Fagg Russell and Sharon Costin, Kirsten Cowley, Lyn Craven (Petraeomyrtus punicea photograph) Copyright © 2013 New Holland Publishers (Australia) Pty Ltd Richard Cummings, Bert