Abies Pinsapo Boiss.: a Protected Species in a Protected Area
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hajar Lamrhari, Mariem Ben-Said, Zineb Jalal, Taoufik Aadel, Hassan
European Scientific Journal, ESJ ISSN: 1857-7881 (Print) e - ISSN 1857-7431 November 2020 edition Vol.16, No.33 Needles in Talassemtane National Park, North-Western Rif Region, Morocco ESJ Natural/Life/Medical Sciences Abstract Moroccan fir, Abies maroccana Trab. Hajar Lamrhari, forms a unique forest community in the Applied Botany Laboratory, Department of Mediterranean basin and has a great Biology, Faculty of Science, University of Abdel ecological and biological values in Malek Essaadi, Tetouan-Morocco Moroccan Rif Mountains. However, Mariem Ben-Said, morphological and anatomical characters Applied Biology and Pathology Laboratory, of the needles are poorly investigated for Department of Biology, Faculty of Science, A. maroccana. This research examined the University of Abdel Malek Essaadi, Tetouan- Morocco morphological and anatomical characters Zineb Jalal, of Moroccan fir needles in order to Physiology Pharmacology and Environmental determine the phenotypic needles traits of Health Laboratory, Department of Biology, Faculty Abies maroccana. The study was carried of Sciences Dhar El Mehraz, University of Sidi out in the National Park of Talassemtane, Mohamed Ben Abdellah,.Atlas, Fez – Western Rif, Morocco. Data was collected Morocco from two-year-old needles in six stands. In Taoufik Aadel, each site, seven trees were selected, and 60 Bureau of Forest Health, Forest Climate Risk Management Center. Department of Water and needles were collected from each tree. Forests. Agdal. Rabat Five morphological and one anatomical Hassan Bouziane, characters of the needles were examined. Applied Botany Laboratory, Department of The variance analysis (ANOVA) of Biology, Faculty of Science, University of Abdel quantitative needles variables revealed Malek Essaadi, Tetouan-Morocco significant inter-tree morphological variability of needles of A. -

Abies Pinsapo Forests in Spain and Morocco: Threats and Conservation
Abies pinsapo forests in Spain and Morocco: threats and conservation L.G. ESTEBAN,P.DE P ALACIOS and L. Rodr´I GUEZ-LOSADA A GUADO Abstract The conifer forests of the Mediterranean Basin inappropriate silvicultural practices continue to threaten have been subjected to overuse by humans since ancient Abies cilicica (Fontaine et al., 2007). In Lebanon 10 conifer times. Some species have survived in inaccessible refuges species are threatened to varying degrees by fragmentation but the ranges of other species have been greatly reduced and deterioration of their habitat (Talhouk et al., 2001). by the effects of clearance for agriculture, livestock raising, Elsewhere in the eastern Mediterranean Abies nordmanni- illegal felling and, in some cases, fire. The firs are no ana subsp. equi-trojani, Abies borisii-regis and some pop- exception and some now exist only as relict species. Abies ulations of Pinus heldreichii require management plans to pinsapo is an example, with the species surviving in only guarantee their survival, as do the populations of Cedrus three enclaves in southern Spain and two in northern brevifolia in Cyprus (Que´zel & Barbero, 1990). Most of the Morocco. Until the mid 20th century A. pinsapo forests conifer forests in the southern Mediterranean are threat- were subject to major anthropogenic pressures, and in ened as a result of deforestation and overgrazing (Barbero Spain they were under constant threat of overuse until they et al., 1990). In the Maghreb of Morocco there is particular were acquired by the State. Conservation efforts have now, concern for Abies numidica, Abies pinsapo var. tazaotana, however, been undertaken in both Spain and Morocco, and Pinus nigra subsp. -

Official Journal C
Officia^_^ /• /* e e l-f l Journa-wr l"I ISScN 0378 19"69865 Volume 33 of the European Communities 3 A.g.« 1,* Notice No Contents page I Information II Preparatory Acts Commission 90/C 195/01 Supplementary Annexes to the proposal for a Council Directive on the protection of natural and semi-natural habitats and of wild fauna and flora 1 1 3. 8. 90 Official Journal of the European Communities No C 195/1 II (Preparatory Acts) COMMISSION Supplementary Annexes to the proposal for a Council Directive on the protection of natural and semi-natural habitats and of wild fauna and flora COM(90) 59 final (Submitted by the Commission on 14 March 1990 pursuant to the third paragraph of Article 149 of the EEC Treaty) (90/C 195/01) EXPLANATORY NOTE The proposal here presented aims to complete the Annexes of the Council's draft Directive on the protection of natural and semi-natural habitats and of wild fauna and flora, adopted by the Commission on 26 July 1988 (x). (>) OJNoC247, 21. 9. 1988. No C 195/2 Official Journal of the European Communities 3. 8. 90 ANNEX I Animal and plant species whose habitats are threatened in the Community (a) ANIMALS VERTEBRATES MAMMALS Rupicapra rupicapra balcanica Ovis ammon musimon (Natural populations — Corsica and INSECTIVORA Sardinia) Talpidae Capra aegagrus Galemys pyrenaicus CETACEA Soricidae Tursiops truncatus Suncus etruscus Phocoena phocoena CHIROPTERA Rhinolophidae REPTILES Rhinolophus blasii Rhinolophus euryale TESTUDINES Rhinolophus ferrumequinum Testudinidae Rhinolophus hipposideros Testudo hermanni Rhinolophus -

Erfahrungen Mit Abies-Arten in Südwestdeutschland
ERFAHRUNGEN MIT ABIES-ARTEN IN SÜDWESTDEUTSCHLAND Von HUBERTUS NIMSCH Zusammenfassung In den vergangenen Jahrzehnten sind vom Autor Kulturversuche im Arboretum Günterstal, Freiburg, mit über 60 Tannen-Arten und Varietäten aus aller Welt durchgeführt worden. Hier wird in alphabetischer Folge über deren natürliche Verbreitung, die genetische und taxonomische Differenzierung sowie über die bisherigen Kultur-Erfahrungen unter südwestdeutschen Klimabedingungen berichtet. Einführung Eine intensive praktische Beschäftigung mit zahlreichen, z.T. selten kultivierten Arten der Gattung Abies (die zur Familie der Pinaceae, Unterfamilie Abietoideae, gehört) ist der Anlass diese über einen Zeitraum von mehreren Jahrzehnten gesammelten Erfahrungen in knappen Worten zusammen zu tragen. Diese Erfahrungen aus dem Arboretum Freiburg-Günterstal können und sollen nur stichwortartig dargestellt werden. Ebenso stichwortartig soll versucht werden, die jeweilige Abies-Art abrundend mit ihrem korrekten wissenschaftlichen Namen, ihren Synonyma sowie mit ihrem deutschen und englischen Namen (soweit vorhanden) und dem einheimischen Namen darzustellen. Kurz gefasst werden einige Bemerkungen zu den Themen Naturvorkommen, genetische Differenzierung, weiterführende Literatur bzw. und Ökologie gemacht. Zum Thema „Örtliche Erfahrungen“ folgen Aussagen, die sich nur auf den Bereich Freiburg und den Westabfall des Schwarzwaldes beziehen. Erfahrungen an anderen Standorten können deshalb durchaus verschieden oder widersprüchlich sein. Bewusst wird auf eine allgemeine Beschreibung der Tannenarten, auf Standort – und Klimaansprüche, auf Wachstum und Entwicklung, auf Nutzung und Pathologie u.a. verzichtet und stattdessen auf weiterführende Literatur bzw. auf Autorennamen verwiesen. Aus der Reihenfolge der im Schrifttum genannten Autoren ist keine Wertigkeit abzuleiten. Neben der umfassenden Abies-Monographie von LIU (1971) wurde bezüglich der chinesischen Tannenarten den Aussagen von CHENG (1978) und bezüglich der mexikanischen Tannenarten den Aussagen von MARTINEZ (1963) größere Bedeutung beigemessen. -

Contrasting Growth and Water Use Efficiency After Thinning in Mixed Abies Pinsapo–Pinus Pinaster–Pinus Sylvestris Forests
JOURNAL OF FOREST SCIENCE, 62, 2016 (2): 53–64 doi: 10.17221/104/2015-JFS Contrasting growth and water use efficiency after thinning in mixed Abies pinsapo–Pinus pinaster–Pinus sylvestris forests R.M. Navarro-Cerrillo1, R. Sánchez-Salguero2, R. Herrera3, C.J. Ceacero Ruiz4, J.M. Moreno-Rojas5, R.D. Manzanedo6, J. López-Quintanilla7 1Departamento Ingeniería Forestal, DendrodatLab- ERSAF, Universidad de Córdoba, Córdoba, Spain 2Departamento de Ecología, Facultad de Ciencias Experimentales, Universidad Pablo de Olavide, Sevilla, Spain 3Instituto Venezolano de Investigaciones Científicas (IVIC), Caracas, Venezuela 4Departamento Fisiología, Universidad Pablo de Olavide, Sevilla, Spain 5Instituto de Investigación y Formación Agraria y Pesquera (IFAPA), Córdoba, Spain 6Institute of Plant Sciences, University of Bern, Bern, Switzerland 7Consejería Medio-Ambiente y Ordenación del Territorio, Málaga, Spain ABSTRACT: Foresters frequently lack sufficient information about thinning intensity effects to optimize semi-natural forest management and their effects and interaction with climate are still poorly understood. In an Abies pinsapo–Pinus pinaster–Pinus sylvestris forest with three thinning intensities, a dendrochronologial approach was used to evaluate the short-term responses of basal area increment (BAI), carbon isotope (δ13C) and water use efficiency (iWUE) to thinning intensity and climate. Thinning generally increased BAI in all species, except for the heavy thinning in P. sylvestris. Across all the plots, thinning increased 13C-derived water-use efficiency on average by 14.49% for A. pinsapo, 9.78% for P. sylves- tris and 6.68% for P. pinaster, but through different ecophysiological mechanisms. Our findings provide a robust mean of predicting water use efficiency responses from three coniferous species exposed to different thinning strategies which have been modulated by climatic conditions over time. -

Collecting Rare Conifers in North Africa
Collecting Rare Conifers in North Africa ’ Robert G. Nicholson Conifer seeds gathered on mountain peaks in Morocco may yield varieties more cold-hardy than those now in cultivation As the cold came on, and as each more southern zone became fitted for the inhabitants of the north, these would take the places of the former inhabitants of the temperate regions. The latter, at the same time, would travel further and further southward, unless they were stopped by barners, m which case they would pensh. The mountams would become covered with snow and ice and them former Alpine inhabitants would descend to the plams. By the time that the cold had reached its maximum, we should have an arctic fauna and flora, covering the central parts of Europe, as far south as the Alps and Pyrenees, and even stretching mto Spam. -Charles Darwm The Origin of Species Had the master biologist, Charles Darwin, collecting focussed on the native conifers, travelled in North Africa, he might have which are poorly represented in botanical amended his discourse on glaciation to include gardens. It was important that I collect the those lands south of the Pyrenees and Spain. seeds from as high an elevation as possible to For in Morocco and Algeria a number of ensure that the resulting seedlings would be peaks are high enough to have harbored an of maximum hardiness. This meant exten- alpine flora that was spreading from Europe sive travelling and walking, since the isolated during the periods of glaciation. And upon peaks stand far from the major cities and these mountains there live today relict popu- stretch high above the nearest villages. -

WUCOLS List S Abelia Chinensis Chinese Abelia M ? ? M / / Copyright © UC Regents, Davis Campus
Ba Bu G Gc P Pm S Su T V N Botanical Name Common Name 1 2 3 4 5 6 Symbol Vegetation Used in Type WUCOLS List S Abelia chinensis Chinese abelia M ? ? M / / Copyright © UC Regents, Davis campus. All rights reserved. bamboo Ba S Abelia floribunda Mexican abelia M ? M M / / S Abelia mosanensis 'Fragrant Abelia' fragrant abelia ? ? ? ? ? ? bulb Bu S Abelia parvifolia (A. longituba) Schuman abelia ? ? ? M ? ? grass G groundcover GC Gc S Abelia x grandiflora and cvs. glossy abelia M M M M M / perennial* P S Abeliophyllum distichum forsythia M M ? ? ? ? palm and cycad Pm S Abelmoschus manihot (Hibiscus manihot) sunset muskmallow ? ? ? L ? ? T Abies pinsapo Spanish fir L L L / / / shrub S succulent Su T N Abies spp. (CA native and non-native) fir M M M M / / P N Abronia latifolia yellow sand verbena VL VL VL / ? ? tree T P N Abronia maritima sand verbena VL VL VL / ? ? vine V California N native S N Abutilon palmeri Indian mallow L L L L M M S Abutilon pictum thompsonii variegated Chinese lantern M H M M ? ? Sunset WUCOLS CIMIS ET Representative Number climate 0 Region zones** Cities zones* S Abutilon vitifolium flowering maple M M M / ? ? Healdsburg, Napa, North- San Jose, Salinas, Central 14, 15, 16, 17 1, 2, 3, 4, 6, 8 San Francisco, Coastal San Luis Obispo S Abutilon x hybridum & cvs. flowering maple M H M M / / 1 Auburn, Central Bakersfield, Chico, 8, 9, 14 12, 14, 15, 16 Valley Fresno, Modesto, Sacramento S T Acacia abyssinica Abyssinian acacia / ? / ? / L 2 Irvine, Los South Angeles, Santa 22, 23, 24 1, 2, 4, 6 Coastal Barbara, Ventura, -

Wood Anatomy of the Genus Abies Luis García Esteban*, Paloma
IAWA Journal, Vol. 30 (3), 2009: 231–245 WOOD ANATOMY OF THE GENUS ABIES A REVIEW Luis García Esteban*, Paloma de Palacios, Francisco García Fernández and Ruth Moreno Universidad Politécnica de Madrid. Escuela Técnica Superior de Ingenieros de Montes, Departamento de Ingeniería Forestal, Ciudad Universitaria, 28040 Madrid, Spain *Corresponding author [E-mail: [email protected]] SUMMARY The literature on the wood anatomy of the genus Abies is reviewed and discussed, and complemented with a detailed study of 33 species, 1 sub- species and 4 varieties. In general, the species studied do not show diag- nostic interspecific differences, although it is possible to establish differences between groups of species using certain quantitative and quali- tative features. The marginal axial parenchyma consisting of single cells and the ray parenchyma cells with distinctly pitted horizontal walls, nodular end walls and presence of indentures are constant for the genus, although these features also occur in the other genera of the Abietoideae. The absence of ray tracheids in Abies can be used to distinguish it from Cedrus and Tsuga, and the irregularly shaped parenchymatous marginal ray cells are only shared with Cedrus. The absence of resin canals enables Abies to be distinguished from very closely related genera such as Keteleeria and Nothotsuga. The crystals in the ray cells, taxodioid cross-field pitting and the warty layer in the tracheids can be regarded as diagnostic generic features. Key words: Abies, Abietoideae, anatomy, wood. INTRODUCTION The family Pinaceae, with 11 genera and 225 species, is the largest conifer family. The genus Abies, with 48 species and 24 varieties, has the second highest number of species after the genus Pinus (Farjon 2001). -

County-Approved Plant List (Combined)
County-Approved Plant List (Combined) Plant CA. Water Drought- Growth Ever- Resists Eros. Street Trees Climate Bio BOTANICAL NAME COMMON NAME type Native Needs Tolerant Height Spread Rate green Fire(1) Ag-buffer Cntrl ? Zone Retention OTHER West only Abelia floribunda Mexican Abelia sh N av N 3-6' Y No N 8,9,12-24 N Abelia grandiflora Glossy Abelia sh N av N 8'+ Y N 5 thru 24 N Abies bracteata Santa Lucia/ Bristlecone tr Y lo Y 70' 15-20' slow Y No N U 8, 9, 14-21 heat tolerant; good wildlife plant Y Fir Abies concolor White Fir tr Y av N 30-66' slow Y No N U 1-9,14-24 good wildlife plant N Abies pinsapo Spanish Fir tr N lo N 25-50' very slow Y No N U 5-11, 14-24 good wildlife plant N Abies procera Noble Fir tr Y av N 90-200' slow Y No N U 1-7,15-17 good wildlife plant N Abronia latifolia Yellow Sand Verbena gc Y av N 3' F3 N 4, 5, 17, 24 likes sandy soils Y Abronia maritima Red Sand Verbena gc Y lo N 3" F3 N 4, 5, 17, 24 likes sandy soils Y Abronia umbellata Pink Sand Verbena gc Y av N 3' F3 N 4, 5, 17, 24 likes sandy soils Y Abronia villosa Sand Verbena gc Y lo Y F3 N 4, 5, 17, 24 likes sandy soils Y Acacia baileyana Bailey Acacia tr N lo * 20-30' 20-40' fast Y No N Y 7-9,13-24 * somewhat once established; N needs good drainage; fragrant flowers; good inland ; plant with caution around animals Acacia cultriformis Knife Acacia tr N lo * 10-15' 10-15' mod to fast Y No N U 13-24 * once established; Y needs good drainage; plant with caution around animals Acacia dealbata Silver Wattle tr N lo N To 50' 40-50' fast Y No N U# 8,9,14-24 plant -

9520 Abetales De Abies Pinsapo Boiss
1 PRESENTACIÓN 9520 ABETALES DE ABIES PINSAPO BOISS. COORDINADOR Alejandro Valladares 2 BOSQUES / 9520 ABETALES DE ABIES PINSAPO BOIS Esta ficha forma parte de la publicación Bases ecológicas preliminares para la conservación de los tipos de hábitat de interés comunitario en España, promovida por la Dirección General de Medio Natural y Política Forestal (Ministerio de Medio Ambiente, y Medio Rural y Marino). Dirección técnica del proyecto Rafael Hidalgo. Realización y producción Coordinación general Elena Bermejo Bermejo y Francisco Melado Morillo. Coordinación técnica Juan Carlos Simón Zarzoso. Colaboradores Presentación general: Roberto Matellanes Ferreras y Ramón Martínez Torres. Edición: Cristina Hidalgo Romero, Juan Párbole Montes, Sara Mora Vicente, Rut Sánchez de Dios, Juan García Montero, Patricia Vera Bravo, Antonio José Gil Martínez y Patricia Navarro Huercio. Asesores: Íñigo Vázquez-Dodero Estevan y Ricardo García Moral. Diseño y maquetación Diseño y confección de la maqueta: Marta Munguía. Maquetación: Do-It, Soluciones Creativas. Agradecimientos A todos los participantes en la elaboración de las fichas por su esfuerzo, y especialmente a Antonio Camacho, Javier Gracia, Antonio Martínez Cortizas, Augusto Pérez Alberti y Fernando Valladares, por su especial dedicación y apoyo a la dirección y a la coordinación general y técnica del proyecto. Las opiniones que se expresan en esta obra son responsabilidad de los autores y no necesariamente de la Dirección General de Medio Natural y Política Forestal (Ministerio de Medio Ambiente, y Medio Rural y Marino). 3 PRESENTACIÓN La coordinación general del grupo 9 ha sido encargada a la siguiente institución Asociación Española de Ecología Terrestre Coordinador: Alejandro Valladares1. 1Univ. Politécnica de Madrid. Colaboraciones específicas relacionadas con los grupos de especies: Invertebrados: Centro Iberoamericano de la Biodiversidad (CIBIO, Instituto Universitario de Investigación, Universidad de Alicante). -

Abies Pinsapo International Trip
The Conifer Quarterly is the publication of the American Conifer Society Contents 2-3 .................................... President’s Message 4-7 ..............................................Editor’s Corner 8 ...................Central Region Announcement 9 ..............................................Deer Deterrent 10-14 ................................Dwarf Ginkgo Cultivars ............................. Part I: A cluttered landscape 16-20 Powell Gardens’ Conifer Garden: Circle Oak Ranch .................... An ACS Conifer Reference Garden 21-29 .................................. 2012 International Trip: .......................... The Netherlands and Belgium 30-33 ..... A Wasp’s Tale: The Search for Megastigmus .................... on North American Cupressaceae Powell Gardens 34-36 ........................................The Hibbard Broom, ........... the “VW Bug” Broom and ‘Wilson Park’, .......................................... Three Broom Stories 37-39 ..........The Beazie and Michael Larned Garden ..........................for the 2013 National Meeting 40-41 ............................................... Abies pinsapo International Trip 42 ................................ 2013 National Meeting 44-47 ....2012 Northeast Region Annual Meeting 48 ....................................................Directorate Larned Garden The purposes of the American Conifer Society are the development, conservation, and propagation of conifers, with an emphasis on those that are dwarf or unusual, standardization of nomenclature, and education -
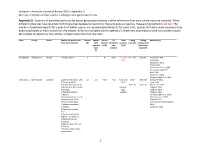
Jacobsen—American Journal of Botany 2021—Appendix S1 Diversity in Conduit and Pit Structure Among Extant Gymnosperm Taxa
Jacobsen—American Journal of Botany 2021—Appendix S1 Diversity in conduit and pit structure among extant gymnosperm taxa Appendix S1. Summary of pit membrane traits for extant gymnosperm genera and the references from which these traits are compiled. When different references have reported conflicting presence/absence reports for the same genus or species, these are highlighted in red text. The number of published reports for a given trait within a genus are included parenthetically for some traits. Species that were newly examined from dried wood blocks or fresh samples for the present review are indicated with an asterisk (*); these new examinations were not counted toward the number of reports nor the numbers of observations for trait characters. Phyla Family Genus Species for which traits Reports Species Vessels Pit Torus Pitting Pitting Reference(s) have been reported (#) with present membrane presence (-seriate) arrangement reported (Y/N) type (Y/N) (alternate/ traits (A/B/C) opposite) (#) Ginkgophyta Ginkgoaceae Ginkgo *Ginkgo biloba L. 9 1 N B (2) Y (1) and uni- & bi- opposite Penhallow 1907, N (6) Liese 1965, Bauch et al. 1972, Timell 1978, Schulte and Gibson 1988, Yin and Xiao-Mei 1992, Dute 1994, Hacke et al. 2004, Schweingruber et al. 2006 Gnetophyta Ephedraceae Ephedra Ephedra americana Humb. 22 12 Y (17) A (2) Y (17) and multi- alternate MacDuffie 1921, & Bonpl. ex Willd., N (2) Liese 1965, Ephedra distachya subsp. (vessels) (vessels) Bauch et al. 1972, helvetica (C.A.Mey.) Asch. (conduit- Carlquist 1990, & Graebn., type Carlquist 1992, Ephedra fasciculata specific) Carlquist 1996, A.Nelson, Schweingruber et al. 2006, Ephedra foeminea Forssk., Motomura et al.