Impatiens Glandulifera
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Germination, Vegetative and Flowering Behavior of Balsam (Impatiens
International Journal of Environment, Agriculture and Biotechnology (IJEAB) Vol-3, Issue -4, Jul-Aug- 2018 http://dx.doi.org/10.22161/ijeab/3.4.38 ISSN: 2456-1878 Germination, vegetative and flowering behavior of Balsam (Impatiens balsamina L.) in response to natural photoperiods Muhammad Aslam Baloch1, Tanveer Fatima Miano*1, Niaz Ahmed Wahocho1, Naheed Akhtar Talpur2, Abdul Qadir Gola1 1Department of Horticulture, Sindh Agriculture University Tandojam, Pakistan 2Department of Soil Science, Sindh Agriculture University Tandojam, Pakistan Corresponding Author: Dr. Tanveer Fatima Miano*, Associate Professor Corresponding Author Email: [email protected] Abstract— A lack of application of photoperiod and light content (46.79 SPAD) was recorded from NP4= 9 hrs intensity to manipulate the growth of current spring (8:00 am-5:00 pm) as compared to seed germination annuals has, in part, been due to the lack of information (63.88%), germination index (0.66 gi) plant height (14.92 identifying the photoperiodic and light intensities cm), leaves plant-1 (48.16), days to 1st flower (45.96), requirements of various species. Present pot experiment flowers plant-1 (5.00), days to flower persistence (11.16), was carried out at Horticulture Garden, Department of weight of single flower (0.62 g), chlorophyll content (36.46 Horticulture, Sindh Agriculture University Tandojam, SPAD) was recorded from NP1= Control (Normal day during spring 2017, which was laid out in a three length). replicated Complete Randomized Design (CRD). Two Keywords— flowering behaviour, natural photoperiods, varieties of balsam (V1= Tom Thumb, V2 = Double Complete Randomized Design. Camcellia) were studied under NP1= Control (Normal day length), NP2=3 hrs (8:00 am- 11:00 am), NP3= 6 hrs (8:00 I. -

Impatiens Glandulifera (Himalayan Balsam) Chloroplast Genome Sequence As a Promising Target for Populations Studies
Impatiens glandulifera (Himalayan balsam) chloroplast genome sequence as a promising target for populations studies Giovanni Cafa1, Riccardo Baroncelli2, Carol A. Ellison1 and Daisuke Kurose1 1 CABI Europe, Egham, Surrey, UK 2 University of Salamanca, Instituto Hispano-Luso de Investigaciones Agrarias (CIALE), Villamayor (Salamanca), Spain ABSTRACT Background: Himalayan balsam Impatiens glandulifera Royle (Balsaminaceae) is a highly invasive annual species native of the Himalayas. Biocontrol of the plant using the rust fungus Puccinia komarovii var. glanduliferae is currently being implemented, but issues have arisen with matching UK weed genotypes with compatible strains of the pathogen. To support successful biocontrol, a better understanding of the host weed population, including potential sources of introductions, of Himalayan balsam is required. Methods: In this molecular study, two new complete chloroplast (cp) genomes of I. glandulifera were obtained with low coverage whole genome sequencing (genome skimming). A 125-year-old herbarium specimen (HB92) collected from the native range was sequenced and assembled and compared with a 2-year-old specimen from UK field plants (HB10). Results: The complete cp genomes were double-stranded molecules of 152,260 bp (HB92) and 152,203 bp (HB10) in length and showed 97 variable sites: 27 intragenic and 70 intergenic. The two genomes were aligned and mapped with two closely related genomes used as references. Genome skimming generates complete organellar genomes with limited technical and financial efforts and produces large datasets compared to multi-locus sequence typing. This study demonstrates the 26 July 2019 Submitted suitability of genome skimming for generating complete cp genomes of historic Accepted 12 February 2020 Published 24 March 2020 herbarium material. -

Urtica Dioica Stinging Nettle
TREATMENT OPTIONS from the book Weed Control in Natural Areas in the Western United States This does not constitute a formal recommendation. When using herbicides always read the label, and when in doubt consult your farm advisor or county agent. This is an excerpt from the book Weed Control in Natural Areas in the Western United States and is available wholesale through the UC Weed Research & Information Center (wric.ucdavis.edu) or retail through the Western Society of Weed Science (wsweedscience.org) or the California Invasive Species Council (cal-ipc.org). Urtica dioica Stinging nettle Family: Urticaceae (nettle) NON-CHEMICAL CONTROL Cultural: grazing P not readily grazed by livestock Cultural: prescribed burning P below ground structures not affected Mechanical: mowing and cutting P regrows rapidly if mowed early in the growing season Mechanical: tillage P─F likely requires repeated tillage for several years Mechanical: grubbing, digging or hand P─F extensive rhizomes and stinging hairs make hand pulling pulling difficult CHEMICAL CONTROL The following specific use information is based on published papers and reports by researchers and land managers. Other trade names may be available, and other compounds also are labeled for this weed. Directions for use may vary between brands; see label before use. 2,4-D E Imazapic NIA Aminocyclopyrachlor + chlorsulfuron NIA Imazapyr G Aminopyralid E Metsulfuron NIA Chlorsulfuron NIA Paraquat P Clopyralid P Picloram E Dicamba E Rimsulfuron NIA Glyphosate E Sulfometuron NIA Hexazinone NIA Sulfosulfuron NIA Triclopyr E E = Excellent control, generally better than 95% * = Likely based on results of observations of G = Good control, 80-95% related species FLW = flowering F = Fair control, 50-80% NIA = No information available P = Poor control, below 50% Fa = Fall Control includes effects within the season of treatment. -

Native Stinging Nettle (Urtica Dioica
OATAO is an open access repository that collects the work of Toulouse researchers and makes it freely available over the web where possible This is an author’s version published in: http://oatao.univ-toulouse.fr/25522 Official URL: https://doi.org/10.1016/j.indcrop.2019.111997 To cite this version: Jeannin, Thomas and Yung, Loïc and Evon, Philippe and Labonne, Laurent and Ouagne, Pierre and Lecourt, Michael and Cazaux, David and Chalot, Michel and Placet, Vincent Native stinging nettle (Urtica dioica L.) growing spontaneously under short rotation coppice for phytomanagement of trace element contaminated soils: Fibre yield, processability and quality. (2020) Industrial Crops and Products, 145. 1119997. ISSN 0926-6690 Any correspondence concerning this service should be sent to the repository administrator: [email protected] Native stinging nettle ( Urtica dioica L.) growing spontaneously under short rotation coppice forphytomanagement of trace element contaminated soils: Fibre yield, processability and quality 3 b C C d Thomas Jeannin , Loïc Yung , Philippe Evon , Laurent Labonne , Pierre Ouagne , Michael Lecourte, David Cazamr, Michel Chalotb, Vincent Placet'•'" • PEMTO-STlnsdlutt, UPC/CNRS!ENSMM/UTBM, Uni1<ersitiBo,rgogne Promhe.-Comti, Besançon, Prome b LaborotOin Chrono-Environnement, UMR CNRS/UPC, UniversitiBowwgne Pranche-Comli, Montbéliard, Prome • LaboratOire de ChimieAi,oo-lndustrlelle (LCA), INP-ENSIACET, Toulouse, France • LaborotOire Géniede Production (U::P), Universitide Toulouse-ENIT, Tarbes, Prome • -

Invasive Species Identification Guide
Invasive Species Identification guides – Table of Contents Floating Pennywort (Hydrocotyle ranunculoides)……………………………….…………………………………2 Giant Hogweed (Heracleum mantegazzianum)…………………………………………………………………….4 Himalayan balsam (Impatiens glandulifera)………………………………………………………….…………….6 Japanese knotweed (Fallopia japonica)………………………………………………………………………………..8 New Zealand pigmyweed (Crassula helmsii)……………………………………………………………………….10 Water fern (Azolla filiculoides)……………………………………………………………………………………………12 This list is by no means exhaustive. Please refer to pages 233-237 of the Lincolnshire Biodiversity Action Plan (3rd Edition) for a list of priority non-native invasive species for our area. Floating pennywort Hydrocotyle ranunculoides Invasive species identification guide Where is it found? Floating on the surface or emerging from still or slowly moving freshwater. Can be free floating or rooted. Similar to…… Marsh pennywort (see bottom left photo) – this has a smaller more rounded leaf that attaches to the stem in the centre rather than between two lobes. Will always be rooted and never free floating. Key features Lobed leaves Fleshy green or red stems Floating or rooted Forms dense interwoven mats Leaf Leaves can be up to 7cm in diameter. Lobed leaves can float on or stand above the water. Marsh pennywort Roots Up to 5cm Fine white Stem attaches in roots centre More rounded leaf Floating pennywort Larger lobed leaf Stem attaches between lobes Photos © GBNNSS Workstream title goes here Invasive species ID guide: Floating pennywort Management Floating pennywort is extremely difficult to control due to rapid growth rates (up to 20cm from the bank each day). Chemical control: Can use glyphosphate but plant does not rot down very quickly after treatment so vegetation should be removed after two to three weeks in flood risk areas. Regular treatment necessary throughout the growing season. -

Regulation of Urtica Dioica L. on Grasslands Vozár Ľ., Jančovič J
Regulation of Urtica dioica L. on grasslands Vozár Ľ., Jančovič J. and Bačová S. Slovak Agricultural University in Nitra, Faculty of Agrobiology and Food Resources, Department of Grassland Ecosystems and Forage Crops, Nitra, Slovakia E-mail: [email protected] Abstract We studied some possibilities for the biological and mechanical control of the ground cover of Galio-Urticetea Passarge ex Kopecký 1969, with the association of Urtica dioica L., in a place which belongs to the village of Chvojnica in the Strážov Hills in the middle of Slovakia. The place was used as a corral for cattle in the past. Cattle had a great influence on the eutrophication of the soil. The floristic composition changed there. We examined 4 variants in our experiment: 1st – control, without cutting; 2nd – cutting every 5th week with biomass being taken away; 3rd – cutting every 5th week with mulch- ing; 4th –Dactylis glomerata L. and Trifolium repens L. reseeding, cutting four times a year. According to the five-year results of the different types of regulation it seems that the best way of regulation is reseeding with the strong competitive species Dactylis glomerata L. and Trifolium repens L. Keywords: regulation of weed infestation, Urtica dioica L., stand eutrophication, corral for cattle Introduction Livestock numbers have decreased to 1/3 of their original level in Slovakia since the year 1989 (Green Report, 2007). The area of grassland utilisation has been reduced. Inaccessible areas have been abandoned and degraded and have become covered by weeds. These stands have low or toxic forage value and these weeds also have negative influences on the en- vironment and formation of the countryside. -

Maine Coefficient of Conservatism
Coefficient of Coefficient of Scientific Name Common Name Nativity Conservatism Wetness Abies balsamea balsam fir native 3 0 Abies concolor white fir non‐native 0 Abutilon theophrasti velvetleaf non‐native 0 3 Acalypha rhomboidea common threeseed mercury native 2 3 Acer ginnala Amur maple non‐native 0 Acer negundo boxelder non‐native 0 0 Acer pensylvanicum striped maple native 5 3 Acer platanoides Norway maple non‐native 0 5 Acer pseudoplatanus sycamore maple non‐native 0 Acer rubrum red maple native 2 0 Acer saccharinum silver maple native 6 ‐3 Acer saccharum sugar maple native 5 3 Acer spicatum mountain maple native 6 3 Acer x freemanii red maple x silver maple native 2 0 Achillea millefolium common yarrow non‐native 0 3 Achillea millefolium var. borealis common yarrow non‐native 0 3 Achillea millefolium var. millefolium common yarrow non‐native 0 3 Achillea millefolium var. occidentalis common yarrow non‐native 0 3 Achillea ptarmica sneezeweed non‐native 0 3 Acinos arvensis basil thyme non‐native 0 Aconitum napellus Venus' chariot non‐native 0 Acorus americanus sweetflag native 6 ‐5 Acorus calamus calamus native 6 ‐5 Actaea pachypoda white baneberry native 7 5 Actaea racemosa black baneberry non‐native 0 Actaea rubra red baneberry native 7 3 Actinidia arguta tara vine non‐native 0 Adiantum aleuticum Aleutian maidenhair native 9 3 Adiantum pedatum northern maidenhair native 8 3 Adlumia fungosa allegheny vine native 7 Aegopodium podagraria bishop's goutweed non‐native 0 0 Coefficient of Coefficient of Scientific Name Common Name Nativity -
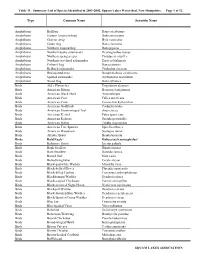
Summary Data
Table 11. Summary List of Species Identified in 2001-2002, Squam Lakes Watershed, New Hampshire. Page 1 of 12 Type Common Name Scientific Name Amphibians Bullfrog Rana catesbeiana Amphibians Eastern American toad Bufo americanus Amphibians Gray treefrog Hyla versicolor Amphibians Green frog Rana clamitans Amphibians Northern leopard frog Rana pipiens Amphibians Northern dusky salamander Desmognathus fuscus Amphibians Northern spring peeper Pseudacris crucifer Amphibians Northern two-lined salamander Eurycea bislineata Amphibians Pickerel frog Rana palustris Amphibians Redback salamander Plethodon cinereus Amphibians Red-spotted newt Notophthalmus viridescens Amphibians Spotted salamander Ambystoma maculatum Amphibians Wood frog Rana sylvatica Birds Alder Flycatcher Empidonax alnorum Birds American Bittern Botaurus lentiginosus Birds American Black Duck Anas rubripes Birds American Coot Fulica americana Birds American Crow Corvus brachyrhynchos Birds American Goldfinch Carduelis tristis Birds American Green-winged Teal Anas crecca Birds American Kestrel Falco sparverius Birds American Redstart Setophaga ruticilla Birds American Robin Turdus migratorius Birds American Tree Sparrow Spizella arborea Birds American Woodcock Scolopax minor Birds Atlantic Brant Branta bernicla Birds Bald Eagle* Haliaeetus leucocephalus* Birds Baltimore Oriole Icterus galbula Birds Bank Swallow Riparia riparia Birds Barn Swallow Hirundo rustica Birds Barred Owl Strix varia Birds Belted Kingfisher Ceryle alcyon Birds Black-and-white Warbler Mniotilta varia Birds -

Deep Roots Radio 2020 Spring Herbs 1
RED CLOVER HERBAL APOTHECARY FARM SPRING HERBS Stinging Nettles (Urtica dioica) leaf, root and seeds One of my very favorite herbs • Use as a tea or in plant extracts. Use as a food - saute in olive oil with garlic, add to pasta dishes, make Nettle leaf lasagna. Use the dried seeds at end of growing season • Stinging nettle is a powerful source of many nutrients, calcium, manganese, magnesium, vitamin K, carotenoids, and protein • Because of its high mineral content, Nettle strengthens bones, hair, nails, and teeth. • Nettles build red blood cells so boosts iron levels. Many women this instead of iron pills when pregnant. Restoring iron levels can also relieve fatigue. • Nettles natural histamine may decrease seasonal allergic responses, such as hay fever. Suggested use for allergies - make a strong nourishing infusions (1 ounce of nettle leaf by weight, infused in 32 ounces of just-boiled water for 4-8 hours) Drink this daily in the time leading up to allergy season. This often eliminates or strongly decreases allergy symptoms • There's the old tale of Elders running naked through the Nettle patches. Stinging nettle hairs contain several chemicals that have pain-relieving and anti-inflammatory properties. This means that stinging nettle could help reduce pain and inflammation in conditions such as arthritis. • Nettle stings contain histamine that causes the initial reaction when you are stung. Dock leaf sap contains natural antihistamine, which helps to ease the stinging sensation. • Stinging Nettle seed is currently being used in clinical practice as a trophorestorative for degenerative kidney disease. A trophorestorative nourishes and restores the function of a tired, compromised, diseased tissue, organ, or system. -

As an Aqueous Plant-Based Extract Fertilizer in Green Bean (Phaseolus Vulgaris L.) Sustainable Agriculture
sustainability Article Stinging Nettle (Urtica dioica L.) as an Aqueous Plant-Based Extract Fertilizer in Green Bean (Phaseolus vulgaris L.) Sustainable Agriculture Branka Mariˇci´c 1,*, Sanja Radman 2, Marija Romi´c 2, Josipa Perkovi´c 3 , Nikola Major 3 , Branimir Urli´c 4, Igor Palˇci´c 3,* , Dean Ban 3, Zoran Zori´c 5 and Smiljana Goreta Ban 3 1 Department of Ecology, Agronomy and Aquaculture, University of Zadar, 23000 Zadar, Croatia 2 Faculty of Agriculture, University of Zagreb, 10000 Zagreb, Croatia; [email protected] (S.R.); [email protected] (M.R.) 3 Institute of Agriculture and Tourism, Department of Agriculture and Nutrition, 52440 Poreˇc,Croatia; [email protected] (J.P.); [email protected] (N.M.); [email protected] (D.B.); [email protected] (S.G.B.) 4 Institute for Adriatic Crops and Karst Reclamation, Department of Plant Sciences, 21000 Split, Croatia; [email protected] 5 Faculty of Food Technology and Biotechnology, University of Zagreb, 10000 Zagreb, Croatia; [email protected] * Correspondence: [email protected] (B.M.); [email protected] (I.P.); Tel.: +385-98-981-7375 (B.M.); +385-408-312 (I.P.) Abstract: Plant-based fertilizers, such as liquid plant extracts, contribute to the cultivation of veg- etables, particularly in organic production. The objective of this study was to determine if aqueous nettle extract could be successfully used as a fertilizer, applied on the soil and foliarly, in green bean Citation: Mariˇci´c,B.; Radman, S.; production under field conditions. The hypothesis was that it could successfully replace mineral Romi´c,M.; Perkovi´c,J.; Major, N.; fertilizers and be integrated into sustainable and organic agriculture. -

Urtica Urens
Dwarf Nettle Urtica urens Weed management guide for Australian vegetable production INTEGRATED WEED MANAGEMENT Identification Dwarf nettle (Urtica urens) is an annual herbaceous plant, native to Mediterranean Europe, that grows between 10 and 75 cm in height. Leaves are up to 6 cm in length but often 1-3 cm, oval to elliptical in shape, deeply toothed or serrated on the edges, green to dark green, and covered with scattered stinging hairs. Clusters of small, greenish-white flowers form where 1 the leaves join the stems. Dwarf nettle is also known in Australia as small nettle, lesser nettle, or stinging nettle. Vegetable farmers are likely to be very familiar with it where it is found on their farm, and to be well aware of how to identify it. However depending on its stage of growth, it may be possible to mis-identify it as tall nettle (Urtica dioica), native scrub 2 nettle (Urtica incisa) or potentially deadnettle (Lamium amplexicaule), particularly where the plants are recently germinated. Figure 1 includes a series of photos of dwarf nettle at different life stages in order to facilitate correct identifica- tion on-farm, from a young seedling through to a mature 3 flowering plant. Dwarf nettle may be mis-identified as native scrub nettle (Urtica incisa), a common species native to Australia. 4 Compared to dwarf nettle, native scrub nettle reaches up to 1m in height, has longer lance-shaped leaves (up to 12 cm) that are paler beneath, fewer stinging hairs, and spike-like clusters of flowers that can be longer than the leaf stalks. -

How Does Genome Size Affect the Evolution of Pollen Tube Growth Rate, a Haploid Performance Trait?
Manuscript bioRxiv preprint doi: https://doi.org/10.1101/462663; this version postedClick April here18, 2019. to The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv aaccess/download;Manuscript;PTGR.genome.evolution.15April20 license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Effects of genome size on pollen performance 2 3 4 5 How does genome size affect the evolution of pollen tube growth rate, a haploid 6 performance trait? 7 8 9 10 11 John B. Reese1,2 and Joseph H. Williams2 12 Department of Ecology and Evolutionary Biology, University of Tennessee, Knoxville, TN 13 37996, U.S.A. 14 15 16 17 1Author for correspondence: 18 John B. Reese 19 Tel: 865 974 9371 20 Email: [email protected] 21 1 bioRxiv preprint doi: https://doi.org/10.1101/462663; this version posted April 18, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 22 ABSTRACT 23 Premise of the Study – Male gametophytes of most seed plants deliver sperm to eggs via a 24 pollen tube. Pollen tube growth rates (PTGRs) of angiosperms are exceptionally rapid, a pattern 25 attributed to more effective haploid selection under stronger pollen competition. Paradoxically, 26 whole genome duplication (WGD) has been common in angiosperms but rare in gymnosperms.