SUMMER WILDFLOWERS Late May, June, July, Early August (96 Species)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Moringa Oleifera 31.05.2005 8:55 Uhr Seite 1
Moringa oleifera 31.05.2005 8:55 Uhr Seite 1 Moringa oleifera III-4 Moringa oleifera LAM., 1785 syn.: Guilandina moringa LAM.; Hyperanthera moringa WILLD.; Moringa nux-ben PERR.; Moringa pterygosperma GAERTN., 1791 Meerrettichbaum, Pferderettichbaum Familie: Moringaceae Arabic: rawag Malayalam: murinna, sigru Assamese: saijna, sohjna Marathi: achajhada, shevgi Bengali: sajina Nepali: shobhanjan, sohijan Burmese: daintha, dandalonbin Oriya: sajina Chinese: la ken Portuguese: moringa, moringueiro English: drumstick tree, Punjabi: sainjna, soanjna horseradish tree, ben tree Sanskrit: shobhanjana, sigru French: moringe à graine ailée, Sinhalese: murunga morungue Spanish: ángela, ben, moringa Gujarati: midhosaragavo, saragavo Swahili: mrongo, mzunze Hindi: mungna, saijna, shajna Tamil: moringa, murungai Kannada: nugge Telegu: mulaga, munaga, Konkani: maissang, moring, tellamunaga moxing Urdu: sahajna Fig. 1: Flower detail (front and side view) Enzyklopädie der Holzgewächse – 40. Erg.Lfg. 6/05 1 Moringa oleifera 31.05.2005 8:55 Uhr Seite 2 Moringa oleifera III-4 Drumstick tree, also known as horseradish tree and ben It is cultivated and has become naturalized in other parts tree in English, is a small to medium-sized, evergreen or of Pakistan, India, and Nepal, as well as in Afghanistan, deciduous tree native to northern India, Pakistan and Bangladesh, Sri Lanka, Southeast Asia, West Asia, the Nepal. It is cultivated and has become naturalized well Arabian peninsula, East and West Africa, throughout the beyond its native range, including throughout South Asia, West Indies and southern Florida, in Central and South and in many countries of Southeast Asia, the Arabian Pe- America from Mexico to Peru, as well as in Brazil and ninsula, tropical Africa, Central America, the Caribbean Paraguay [17, 21, 29, 30, 51, 65]. -
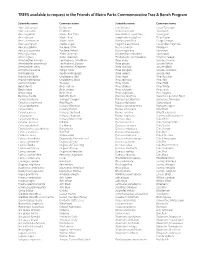
TREES Available to Request in the Friends of Metro Parks Commemorative Tree & Bench Program
TREES available to request in the Friends of Metro Parks Commemorative Tree & Bench Program Scientific name Common name Scientific name Common name Abies balsamaea Fir, Balsam Larix laricina Larch, Tamarack Abies concolor Fir, White Lindera benzoin Spicebush Acer negundo Maple, Box Elder Liquidamber styraciflua Sweetgum Acer rubrum Maple, Red Liriodendron tulipfera Tulip Poplar Acer saccharinum Maple, Silver Maclura pomifera Osage Orange Acer saccharum Maple, Sugar Magnolia acuminata Cucumber magnolia Aesculus glabra Buckeye, Ohio Nyssa sylvatica Blackgum Aesculus octandra Buckeye, Yellow Ostrya viginiana Ironwood Alnus glutinosa Alder, Common Oxydendron arboream Sourwood Alnus rugosa Alder, Speckled Parthenosisis quinquefolia Virginia Creeper Amelanchier arborea Serviceberry, Shadblow Picea abies Spruce, Norway Amelanchier canadensis Serviceberry, Downy Picea glauca Spruce, White Amelanchier laevis Serviceberry Allegheny Picea mariana Spruce, Black Amopha frutocosa Indigo, False Picea pungens Spruce, Colorado Aralia spinosa Devils-walkingstick Picea rubens Spruce, Red Aronia arbutifolia Chokeberry, Red Pinus nigra Pine, Austrian Aronia melancarpa Chokeberry, Black Pinus resinosa Pine, Red Asimina triloba Pawpaw Pinus rigida Pine, Pitch Betula lenta Birch, Yellow Pinus strobus Pine, White Betula lutea Birch, Sweet Pinus sylvestris Pine, Scots Betula nigra Birch, River Pinus virginiana Pine, Virginia Buddeia davidii Butterfly Bush Platanus acerifolia Sycamore, London Plane Campsis radicans Trumpet Creeper Platanus occidentalis Sycamore, -

Asparagus
Give Your Family More of the Good Stuff! Asparagus Basics $ and $ n excellent sourc hop ave is a e of V gus ita < Look for stalks that are firm ra ps build stro m a hel ng bo in with tightly closed tips. Color sp ich ne K A wh s. , can be bright green, creamy white or even purple. < Stalks with the same thickness will cook in the same amount of time. < Fresh asparagus may be best Types of quality and lowest price when harvested locally, usually April Asparagus and May. Generally, thinner spears are < Asparagus is also available more delicate and tender; canned and frozen. thicker spears have stronger flavor and hearty texture. Asparagus Math: Thicker spears can be sliced on the diagonal into smaller One pound = 12 to 15 spears, pieces to cook more quickly. 9 to 10 inches long and 1/2 < Green – the most common to 3/4 inches thick type. = 3 cups trimmed < White – covered with soil as it grows to keep green 1 = 2 /2 cups cooked pigments from developing. Considered a delicacy and may cost more than green. tore Well < Purple – has more sugar and S less fiber than green. The skin aste Less is burgundy or purple but the W flesh is pale green to creamy I Refrigerate fresh asparagus for up white. Cooking may cause I to 5 days. Wash under cool running water more green to show. Less • Stand stalks in 1 inch of water just before using. Remove tough ends: commonly available and may like a flower bouquet and cover • Hold an end of the stalk in each cost more than green. -

Verticillium Wilt of Vegetables and Herbaceous Ornamentals
Dr. Sharon M. Douglas Department of Plant Pathology and Ecology The Connecticut Agricultural Experiment Station 123 Huntington Street, P. O. Box 1106 New Haven, CT 06504 Phone: (203) 974-8601 Fax: (203) 974-8502 Founded in 1875 Email: [email protected] Putting science to work for society Website: www.ct.gov/caes VERTICILLIUM WILT OF VEGETABLES AND HERBACEOUS ORNAMENTALS Verticillium wilt is a disease of over 300 SYMPTOMS AND DISEASE species throughout the United States. This DEVELOPMENT: includes a wide variety of vegetables and Symptoms of Verticillium wilt vary by host herbaceous ornamentals. Tomatoes, and environmental conditions. In many eggplants, peppers, potatoes, dahlia, cases, symptoms do not develop until the impatiens, and snapdragon are among the plant is bearing flowers or fruit or after hosts of this disease. Plants weakened by periods of stressful hot, dry weather. Older root damage from drought, waterlogged leaves are usually the first to develop soils, and other environmental stresses are symptoms, which include yellowing, thought to be more prone to infection. wilting, and eventually dying and dropping from the plant. Infected leaves can also Since Verticillium wilt is a common disease, develop pale yellow blotches on the lower breeding programs have contributed many leaves (Figure 1) and necrotic, V-shaped varieties or cultivars of plants with genetic lesions at the tips of the leaves. resistance—this has significantly reduced the prevalence of this disease on many plants, especially on vegetables. However, the recent interest in planting “heirloom” varieties, which do not carry resistance genes, has resulted in increased incidence of Verticillium wilt on these hosts. -

Urtica Dioica Stinging Nettle
TREATMENT OPTIONS from the book Weed Control in Natural Areas in the Western United States This does not constitute a formal recommendation. When using herbicides always read the label, and when in doubt consult your farm advisor or county agent. This is an excerpt from the book Weed Control in Natural Areas in the Western United States and is available wholesale through the UC Weed Research & Information Center (wric.ucdavis.edu) or retail through the Western Society of Weed Science (wsweedscience.org) or the California Invasive Species Council (cal-ipc.org). Urtica dioica Stinging nettle Family: Urticaceae (nettle) NON-CHEMICAL CONTROL Cultural: grazing P not readily grazed by livestock Cultural: prescribed burning P below ground structures not affected Mechanical: mowing and cutting P regrows rapidly if mowed early in the growing season Mechanical: tillage P─F likely requires repeated tillage for several years Mechanical: grubbing, digging or hand P─F extensive rhizomes and stinging hairs make hand pulling pulling difficult CHEMICAL CONTROL The following specific use information is based on published papers and reports by researchers and land managers. Other trade names may be available, and other compounds also are labeled for this weed. Directions for use may vary between brands; see label before use. 2,4-D E Imazapic NIA Aminocyclopyrachlor + chlorsulfuron NIA Imazapyr G Aminopyralid E Metsulfuron NIA Chlorsulfuron NIA Paraquat P Clopyralid P Picloram E Dicamba E Rimsulfuron NIA Glyphosate E Sulfometuron NIA Hexazinone NIA Sulfosulfuron NIA Triclopyr E E = Excellent control, generally better than 95% * = Likely based on results of observations of G = Good control, 80-95% related species FLW = flowering F = Fair control, 50-80% NIA = No information available P = Poor control, below 50% Fa = Fall Control includes effects within the season of treatment. -

Native Stinging Nettle (Urtica Dioica
OATAO is an open access repository that collects the work of Toulouse researchers and makes it freely available over the web where possible This is an author’s version published in: http://oatao.univ-toulouse.fr/25522 Official URL: https://doi.org/10.1016/j.indcrop.2019.111997 To cite this version: Jeannin, Thomas and Yung, Loïc and Evon, Philippe and Labonne, Laurent and Ouagne, Pierre and Lecourt, Michael and Cazaux, David and Chalot, Michel and Placet, Vincent Native stinging nettle (Urtica dioica L.) growing spontaneously under short rotation coppice for phytomanagement of trace element contaminated soils: Fibre yield, processability and quality. (2020) Industrial Crops and Products, 145. 1119997. ISSN 0926-6690 Any correspondence concerning this service should be sent to the repository administrator: [email protected] Native stinging nettle ( Urtica dioica L.) growing spontaneously under short rotation coppice forphytomanagement of trace element contaminated soils: Fibre yield, processability and quality 3 b C C d Thomas Jeannin , Loïc Yung , Philippe Evon , Laurent Labonne , Pierre Ouagne , Michael Lecourte, David Cazamr, Michel Chalotb, Vincent Placet'•'" • PEMTO-STlnsdlutt, UPC/CNRS!ENSMM/UTBM, Uni1<ersitiBo,rgogne Promhe.-Comti, Besançon, Prome b LaborotOin Chrono-Environnement, UMR CNRS/UPC, UniversitiBowwgne Pranche-Comli, Montbéliard, Prome • LaboratOire de ChimieAi,oo-lndustrlelle (LCA), INP-ENSIACET, Toulouse, France • LaborotOire Géniede Production (U::P), Universitide Toulouse-ENIT, Tarbes, Prome • -

Trumpet Creeper (Campsis Radicans) Control Herbicide Options
Publication 20-86C October 2020 Trumpet Creeper (Campsis radicans) Control Herbicide Options Dr. E. David Dickens, Forest Productivity Professor; Dr. David Clabo, Forest Productivity Professor; and David J. Moorhead, Emeritus Silviculture Professor; UGA Warnell School of Forestry and Natural Resources BRIEF Trumpet creeper (Campsis radicans), also known as cow itch vine, trumpet vine, or hummingbird vine, is in the Bignoniaceae family and is native to the eastern United States. Trumpet creeper is frequently found in a variety of southeastern United States forests and can be a competitor in pine stands. If not controlled, it can kill the trees it grows on by canopying over the crowns and not allowing adequate sunlight to get to the tree’s foliage for photo- synthesis. Trumpet creeper is a deciduous, woody vine that can “climb” trees up to 40 feet or greater heights (Photo 1) or form mats on shrubs or grows in clumps lower to the ground (Photo 2). The 1 to 4 inch long green leaves are pinnate, ovate in shape and opposite (Photo 3). The orange to red showy flowers are terminal cymes of 4 to 10 found on the plants during late spring into summer (Photo 4). Large (3 to 6 inches long) seed pods are formed on mature plants in the fall that hold hundreds of seeds (Photo 5). Trumpet creeper control is best performed during active growth periods from mid-June to early October in Georgia. If trumpet creeper has climbed up into a num- ber of trees, a prescribed burn or cutting the vines to groundline may be needed to get the climbing vine down to groundline where foliar active herbicides will be effective. -

Anthracene Derivatives in Some Species of Rumex L
Vol. 76, No. 2: 103-108, 2007 ACTA SOCIETATIS BOTANICORUM POLONIAE 103 ANTHRACENE DERIVATIVES IN SOME SPECIES OF RUMEX L. GENUS MAGDALENA WEGIERA1, HELENA D. SMOLARZ1, DOROTA WIANOWSKA2, ANDRZEJ L. DAWIDOWICZ2 1 Departament of Pharmaceutical Botany Skubiszewski Medical University of Lublin Chodki 1, 20-039 Lublin, Poland e-mail: [email protected] 2 Faculty of Chemistry, Marii Curie-Sk³odowska University of Lublin (Received: June 1, 2006. Accepted: September 5, 2006) ABSTRACT Eight anthracene derivatives (chrysophanol, physcion, emodin, aloe-emodin, rhein, barbaloin, sennoside A and sennoside B) were signified in six species of Rumex L genus: R. acetosa L., R. acetosella L., R. confertus Willd., R. crispus L., R. hydrolapathum Huds. and R. obtusifolius L. For the investigations methanolic extracts were pre- pared from the roots, leaves and fruits of these species. Reverse Phase High Performance Liquid Chromatography was applied for separation, identification and quantitative determination of anthracene derivatives. The identity of these compounds was further confirmed with UV-VIS. Received data were compared. The roots are the best organs for the accumulation of anthraquinones. The total amount of the detected compo- unds was the largest in the roots of R. confertus (163.42 mg/g), smaller in roots R. crispus (25.22 mg/g) and the smallest in roots of R. hydrolapathum (1.02 mg/g). KEY WORDS: Rumex sp., roots, fruits, leaves, anthracene derivatives, RP-HPLC. INTRODUCTION scion-anthrone, rhein, nepodin, nepodin-O-b-D-glycoside and 1,8-dihydroxyanthraquinone from R. acetosa (Dedio The species belonging to the Rumex L. genus are wide- 1973; Demirezer and Kuruuzum 1997; Fairbairn and El- spread in the world. -

Asparagus Densiflorus 'Sprengeri'
FPS051 Asparagus densiflorus ‘Sprengeri’ Sprengeri Asparagus Fern1 Edward F. Gilman, Ryan W. Klein, and Gail Hansen2 Introduction ‘Sprengeri’ Asparagus Fern is a rounded herbaceous perennial that is used in the landscape for its attractive, fine-textured foliage. This 1 to 4 foot-tall plant has true leaves that are scale-like and inconspicuous. The structures that most refer to as leaves are actually leaf-like branchlets called cladophylls. These tiny cladophylls are linear, flat- tened structures that are bright green in color. They occur singly or in groups of 3 or more at a node. The stems of this plant emerge directly from the ground and become woody and spiny, so be careful when handling this species. The thorns cause significant irritation to many people Figure 1. Full form—Asparagus densiflorus: ‘Sprengeri’ Sprengeri that handle the plant. Pretty, red, ovoid berries occur on asparagus fern. Asparagus densiflorus throughout the year. Several birds eat Credits: Edward F. Gilman, UF/IFAS and probably distribute the fruit. These fruits follow tiny, General Information white, flowers that occur in axillary racemes; the flowers are inconspicuous for the most part but fragrant. Seeds Scientific name: Asparagus densiflorus ‘Sprengeri’ germinate in the landscape and the plant has escaped into Pronunciation: ass-SPAR-uh-gus den-sif-FLOR-us natural habitats in parts of Florida. It can also become a Common name(s): ‘Sprengeri’ asparagus fern weed in your landscape. Family: Liliaceae Plant type: herbaceous; perennial USDA hardiness zones: 9B through 11 (Figure 2) Planting month for zone 7: year round Planting month for zone 8: year round Planting month for zone 9: year round Planting month for zone 10 and 11: year round Origin: not native to North America Invasive potential: potentially invasive 1. -

COVER CROPS and SOIL-BORNE FUNGI DANGEROUS TOWARDS the CULTIVATION of SALSIFY (Tragopogon Porrifolius Var
Acta Sci. Pol., Hortorum Cultus 10(2) 2011, 167-181 COVER CROPS AND SOIL-BORNE FUNGI DANGEROUS TOWARDS THE CULTIVATION OF SALSIFY (Tragopogon porrifolius var. sativus (Gaterau) Br.) Elbieta Patkowska, Mirosaw Konopiski University of Life Sciences in Lublin Abstract. Salsify has a remarkable taste and nutritious values. It is a rich source of inulin – a glycoside which has a positive effect on human and animal organisms. The paper pre- sents studies on the species composition of soil-borne fungi infecting the roots of Tragopogon porrifolius var. sativus cultivated with the use of oats, tansy phacelia and spring vetch as cover crops. In a field experiment the cover crops formed abundant green mass before winter and it constituted a natural mulch on the surface of the plough land. It was managed in two ways: 1) mixed with the soil as a result of spring ploughing, or 2) mixed with the soil as a result of pre-winter ploughing. The conventional cultivation of salsify, i.e. without cover crops, constituted the control. The studies established the number and health status of four-week-old salsify seedlings and roots with necrotic signs. A laboratory mycological analysis made it possible to determine the quantitative and qualitative composition of fungi infecting the underground parts of Tragopogon porri- folius var. sativus. The emergences and the proportion of infected salsify seedlings varied and depended on the species of the mulching plant. The smallest number of infected seed- lings was obtained after the mulch with oats, slightly more after the application of spring vetch or tansy phacelia as cover crops, and the most in the control. -

Towards Resolving Lamiales Relationships
Schäferhoff et al. BMC Evolutionary Biology 2010, 10:352 http://www.biomedcentral.com/1471-2148/10/352 RESEARCH ARTICLE Open Access Towards resolving Lamiales relationships: insights from rapidly evolving chloroplast sequences Bastian Schäferhoff1*, Andreas Fleischmann2, Eberhard Fischer3, Dirk C Albach4, Thomas Borsch5, Günther Heubl2, Kai F Müller1 Abstract Background: In the large angiosperm order Lamiales, a diverse array of highly specialized life strategies such as carnivory, parasitism, epiphytism, and desiccation tolerance occur, and some lineages possess drastically accelerated DNA substitutional rates or miniaturized genomes. However, understanding the evolution of these phenomena in the order, and clarifying borders of and relationships among lamialean families, has been hindered by largely unresolved trees in the past. Results: Our analysis of the rapidly evolving trnK/matK, trnL-F and rps16 chloroplast regions enabled us to infer more precise phylogenetic hypotheses for the Lamiales. Relationships among the nine first-branching families in the Lamiales tree are now resolved with very strong support. Subsequent to Plocospermataceae, a clade consisting of Carlemanniaceae plus Oleaceae branches, followed by Tetrachondraceae and a newly inferred clade composed of Gesneriaceae plus Calceolariaceae, which is also supported by morphological characters. Plantaginaceae (incl. Gratioleae) and Scrophulariaceae are well separated in the backbone grade; Lamiaceae and Verbenaceae appear in distant clades, while the recently described Linderniaceae are confirmed to be monophyletic and in an isolated position. Conclusions: Confidence about deep nodes of the Lamiales tree is an important step towards understanding the evolutionary diversification of a major clade of flowering plants. The degree of resolution obtained here now provides a first opportunity to discuss the evolution of morphological and biochemical traits in Lamiales. -

Concentrations of Anthraquinone Glycosides of Rumex Crispus During Different Vegetation Stages L
Concentrations of Anthraquinone Glycosides of Rumex crispus during Different Vegetation Stages L. Ömtir Demirezer Hacettepe University, Faculty of Pharmacy, Department of Pharmacognosy, 06100 Ankara, Turkey Z. Naturforsch. 49c, 404-406 (1994); received January 31, 1994 Rumex crispus, Polygonaceae. Anthraquinone, Glycoside The anthraquinone glycoside contents of various parts of Rumex crispus L. (Polygonaceae) in different vegetation stages were investigated by thin layer chromatographic and spectro- photometric methods. The data showed that the percentage of anthraquinone glycoside in all parts of plant increased at each stage. Anthraquinone glycoside content was increased in leaf, stem, fruit and root from 0.05 to 0.40%. from 0.03 to 0.46%. from 0.08 to 0.34%, and from 0.35 to 0.91% respectively. From the roots of R. crispus, emodin- 8 -glucoside, RGA (isolated in our laboratory, its structure was not elucidated), traceable amount of glucofran- gulin B and an unknown glycoside ( R f = 0.28 in ethyl acetate:methanol:water/100:20:10) was detected in which the concentration was increased from May to August. The other parts of plant contained only emodin- 8 -glucoside. Introduction In the present investigation various parts of Rumex L. (Polygonaceae) is one of several Rumex crispus, leaf, stem, fruit and root were ana genera which is characterized by the presence lyzed separately for their anthraquinone glycoside of anthraquinone derivatives. There are about contents, the glycosides in different vegetation 200 species of Rumex in worldwide (Hegi, 1957). stages were detected individually. By this method, Rumex is represented with 23 species and 5 hy translocation of anthraquinone glycosides were brids in Turkey (Davis, 1965) and their roots have also investigated.