Trumpet Creeper (Campsis Radicans) Control Herbicide Options
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
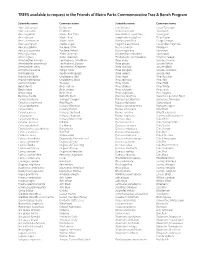
TREES Available to Request in the Friends of Metro Parks Commemorative Tree & Bench Program
TREES available to request in the Friends of Metro Parks Commemorative Tree & Bench Program Scientific name Common name Scientific name Common name Abies balsamaea Fir, Balsam Larix laricina Larch, Tamarack Abies concolor Fir, White Lindera benzoin Spicebush Acer negundo Maple, Box Elder Liquidamber styraciflua Sweetgum Acer rubrum Maple, Red Liriodendron tulipfera Tulip Poplar Acer saccharinum Maple, Silver Maclura pomifera Osage Orange Acer saccharum Maple, Sugar Magnolia acuminata Cucumber magnolia Aesculus glabra Buckeye, Ohio Nyssa sylvatica Blackgum Aesculus octandra Buckeye, Yellow Ostrya viginiana Ironwood Alnus glutinosa Alder, Common Oxydendron arboream Sourwood Alnus rugosa Alder, Speckled Parthenosisis quinquefolia Virginia Creeper Amelanchier arborea Serviceberry, Shadblow Picea abies Spruce, Norway Amelanchier canadensis Serviceberry, Downy Picea glauca Spruce, White Amelanchier laevis Serviceberry Allegheny Picea mariana Spruce, Black Amopha frutocosa Indigo, False Picea pungens Spruce, Colorado Aralia spinosa Devils-walkingstick Picea rubens Spruce, Red Aronia arbutifolia Chokeberry, Red Pinus nigra Pine, Austrian Aronia melancarpa Chokeberry, Black Pinus resinosa Pine, Red Asimina triloba Pawpaw Pinus rigida Pine, Pitch Betula lenta Birch, Yellow Pinus strobus Pine, White Betula lutea Birch, Sweet Pinus sylvestris Pine, Scots Betula nigra Birch, River Pinus virginiana Pine, Virginia Buddeia davidii Butterfly Bush Platanus acerifolia Sycamore, London Plane Campsis radicans Trumpet Creeper Platanus occidentalis Sycamore, -

DESERT WILLOW 'BUBBA' Chilopsis Linearis 'Bubba' Characteristics
DESERT WILLOW ‘BUBBA’ Chilopsis linearis ‘Bubba’ Characteristics Type: Tree Sun: Full sun Zone: 6 to 10 Water: Low to Moderate Height: 25-30 feet Maintenance: Low Spread: 25-30 feet Flower: Showy, Fragrant Bloom Time: Spring through Summer Fruit: Showy Bloom Description: Dark Burgundy and Tolerate: Drought, Dry Soil Pink Texas Native Culture Chilopsis linearis ‘Bubba’ is a vigorous, fast-growing upright selection of desert willow that originated in Texas. Compared to other selections it has a strong vertical form and is less shrubby. It also has glossy, darker green, more lush foliage than other desert willows. It has the capacity to easily grow up to 30 feet tall in the landscape. Starting in late spring and through the summer ‘Bubba’ produces masses of large, fragrant, two-tone burgundy and pink flowers. Like other desert willows ‘Bubba’ is a great choice for full sun, low maintenance, water efficient landscapes. Although it is not seedless, it produces fewer pods than most selections. Each pod containing many winged seeds. Noteworthy Characteristics Chilopsis linearis, commonly known as desert willow, is a large shrub or small multi-trunked tree with a loose open crown. It typically grows to 15-25’ tall with a spread to 10-15’ wide, though some varieties, like ‘Bubba’, grow taller. It is native to gravelly and rocky soils in the Southwestern U.S. and northern Mexico where it is usually found growing in desert grasslands, sandy washes or springs. While the narrow, long leaf shape is indeed willow-like, Chilopsis linearis is in fact related to Catalpa trees, Yellow Bells (Tecoma stans), and Trumpet Vine (Campsis radicans).While oversized in comparison to other members of its family, ‘Bubba’ remains reasonably sized for compact growing spaces as an ornamental accent. -

Podranea Ricasoliana.Pdf
Family: Bignoniaceae Taxon: Podranea ricasoliana Synonym: Pandorea ricasoliana (Tanfani) Baill. Common Name: pink trumpet vine Podranea brycei (N. E. Br.) Sprague Port St. Johns creeper Tecoma brycei N. E. Br. Zimbabwe creeper Tecoma mackenii W. Watson bubblegum-vine Tecoma ricasoliana Tanfani pandorea Questionaire : current 20090513 Assessor: HPWRA OrgData Designation: H(HPWRA) Status: Assessor Approved Data Entry Person: HPWRA OrgData WRA Score 7 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? y=1, n=-1 103 Does the species have weedy races? y=1, n=-1 201 Species suited to tropical or subtropical climate(s) - If island is primarily wet habitat, then (0-low; 1-intermediate; 2- High substitute "wet tropical" for "tropical or subtropical" high) (See Appendix 2) 202 Quality of climate match data (0-low; 1-intermediate; 2- High high) (See Appendix 2) 203 Broad climate suitability (environmental versatility) y=1, n=0 y 204 Native or naturalized in regions with tropical or subtropical climates y=1, n=0 y 205 Does the species have a history of repeated introductions outside its natural range? y=-2, ?=-1, n=0 y 301 Naturalized beyond native range y = 1*multiplier (see y Appendix 2), n= question 205 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see y Appendix 2) 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see n Appendix 2) 304 Environmental weed n=0, y = 2*multiplier (see n Appendix 2) 305 Congeneric weed n=0, y = 1*multiplier (see n Appendix 2) 401 Produces -

Towards Resolving Lamiales Relationships
Schäferhoff et al. BMC Evolutionary Biology 2010, 10:352 http://www.biomedcentral.com/1471-2148/10/352 RESEARCH ARTICLE Open Access Towards resolving Lamiales relationships: insights from rapidly evolving chloroplast sequences Bastian Schäferhoff1*, Andreas Fleischmann2, Eberhard Fischer3, Dirk C Albach4, Thomas Borsch5, Günther Heubl2, Kai F Müller1 Abstract Background: In the large angiosperm order Lamiales, a diverse array of highly specialized life strategies such as carnivory, parasitism, epiphytism, and desiccation tolerance occur, and some lineages possess drastically accelerated DNA substitutional rates or miniaturized genomes. However, understanding the evolution of these phenomena in the order, and clarifying borders of and relationships among lamialean families, has been hindered by largely unresolved trees in the past. Results: Our analysis of the rapidly evolving trnK/matK, trnL-F and rps16 chloroplast regions enabled us to infer more precise phylogenetic hypotheses for the Lamiales. Relationships among the nine first-branching families in the Lamiales tree are now resolved with very strong support. Subsequent to Plocospermataceae, a clade consisting of Carlemanniaceae plus Oleaceae branches, followed by Tetrachondraceae and a newly inferred clade composed of Gesneriaceae plus Calceolariaceae, which is also supported by morphological characters. Plantaginaceae (incl. Gratioleae) and Scrophulariaceae are well separated in the backbone grade; Lamiaceae and Verbenaceae appear in distant clades, while the recently described Linderniaceae are confirmed to be monophyletic and in an isolated position. Conclusions: Confidence about deep nodes of the Lamiales tree is an important step towards understanding the evolutionary diversification of a major clade of flowering plants. The degree of resolution obtained here now provides a first opportunity to discuss the evolution of morphological and biochemical traits in Lamiales. -

Landscape Vines for Southern Arizona Peter L
COLLEGE OF AGRICULTURE AND LIFE SCIENCES COOPERATIVE EXTENSION AZ1606 October 2013 LANDSCAPE VINES FOR SOUTHERN ARIZONA Peter L. Warren The reasons for using vines in the landscape are many and be tied with plastic tape or plastic covered wire. For heavy vines, varied. First of all, southern Arizona’s bright sunshine and use galvanized wire run through a short section of garden hose warm temperatures make them a practical means of climate to protect the stem. control. Climbing over an arbor, vines give quick shade for If a vine is to be grown against a wall that may someday need patios and other outdoor living spaces. Planted beside a house painting or repairs, the vine should be trained on a hinged trellis. wall or window, vines offer a curtain of greenery, keeping Secure the trellis at the top so that it can be detached and laid temperatures cooler inside. In exposed situations vines provide down and then tilted back into place after the work is completed. wind protection and reduce dust, sun glare, and reflected heat. Leave a space of several inches between the trellis and the wall. Vines add a vertical dimension to the desert landscape that is difficult to achieve with any other kind of plant. Vines can Self-climbing Vines – Masonry serve as a narrow space divider, a barrier, or a privacy screen. Some vines attach themselves to rough surfaces such as brick, Some vines also make good ground covers for steep banks, concrete, and stone by means of aerial rootlets or tendrils tipped driveway cuts, and planting beds too narrow for shrubs. -

Ozone Sensitive Plant Species on NPS and U.S. FWS Lands
Ozone Sensitive Plant Species on National Park Service and U.S. Fish and Wildlife Service Lands: Results of a June 24-25, 2003 Workshop Baltimore, Maryland National Park Service Air Resources Division U.S. Fish and Wildlife Service Air Quality Branch November 2003 Ozone Sensitive Plant Species on National Park Service and U.S. Fish and Wildlife Service Lands: Results of a June 24-25, 2003 Workshop Baltimore, Maryland Prepared by: Ellen Porter, Air Resources Division, National Park Service U.S. Department of the Interior National Park Service Air Resources Division, Denver, Colorado U.S. Fish and Wildlife Service Air Quality Branch, Denver, Colorado November 2003 NPS D1522 Natural Resource Report NPS/NRARD/NRR-2003/01 Acknowledgements: Drs. Art Chappelka, Howie Neufeld, Donald Davis, Robert Kohut and Pat Temple provided scientific expertise at the Baltimore Workshop. Dr. Gretchen Smith, Jim Renfro, Dr. David Peterson, Ed Jepsen, Dr. John Skelly, and Dr. William Manning provided additional scientific expertise and peer review. The author wishes to thank Tonnie Maniero, Tamara Blett, and Kristi Morris for helpful editing comments. This report is available at: www2.nature.nps.gov/ard/pubs/index.htm Cover photos by Dr. Donald Davis Contents Summary..................................................................................................1 Background ..............................................................................................1 Workshop Goals and Results .....................................................................3 -

Of the Hunt Institute for Botanical Documentation
Carnegie Mellon University, Pittsburgh, Pennsylvania Vol. 26, No. 1 Bulletin Spring 2014 of the Hunt Institute for Botanical Documentation Inside 4 Duets on display 4 Open House 2014 4 William Andrew 4 Illustrated mushroom Archer papers books, part 2 Above right, Tulipe des jardins. Tulipa gesneriana L. [Tulipa gesnerana Linnaeus, Liliaceae], stipple engraving on paper by P. F. Le Grand, 49 × 32.5 cm, after an original by Gerard van Spaendonck (Holland/France, 1746–1822) for his Fleurs Sessinées d’après Nature (Paris, L’Auteur, au Jardin des Plantes, 1801, pl. 4), HI Art accession no. 2078. Below left, Parrot tulips [Tulipa Linnaeus, Liliaceae], watercolor on paper by Rose Pellicano (Italy/United States), 1998, 56 × 42.5 cm, HI Art accession no. 7405. News from the Art Department Duets exhibition opens The inspiration for the exhibition Duets 1746–1822) has done so with the began with two artworks of trumpet subtle tonality of a monochromatic vine, which were created by the stipple engraving and Rose Pellicano 18th-century, German/English artist (Italy/United States) with rich layers Georg Ehret and the contemporary of watercolor. The former inspired Italian artist Marilena Pistoia. Visitors a legion of botanical artists while frequently request to view a selection teaching at the Jardin des Plantes in of the Institute’s collection of 255 Ehret Paris, and the latter, whose work is and 227 Pistoia original paintings. One inspired by French 18th- and 19th- day we displayed side-by-side the two century artists, carries on this tradition paintings (above left and right) and noticed of exhibiting, instructing and inspiring not only the similarity of composition up-and-coming botanical artists. -

SUMMER WILDFLOWERS Late May, June, July, Early August (96 Species)
SUMMER WILDFLOWERS Late May, June, July, Early August (96 species) Cat-tail Family (Typhaceae) ____ Yellow Sweet Clover ____ Cat-tail (Typha angustifolia) (Melilotus officinalis)* ____ Crimson Clover (Trifolium incarnatum)* Water-plaintain Family (Alismataceae) ____ Red Clover (T. pratense)* ____ Water-plantain (Alisma subcordatum) ____ White Clover (T. repens)* Arum Family (Araceae) Spurge Family (Euphorbiaceae) ___ Jack-in-the-Pulpit (Arisaema atrorubens) ____ Flowering Spurge (Euphorbia corollata) ___ Green Dragon (A. dracontium) Touch-me-not Family (Balsaminaceae) Spiderwort Family (Commelinaceae) ____ Spotted Jewelweed (Impatiens capensis) ____ Virginia Dayflower (Commelina virginica) Mallow Family (Malvaceae) Rush Family (Juncaceae) ____ Common Mallow (Malva neglecta)* ____ Common Rush (Juncus effusus) St. John’s Wort Family (Clusiaceae) Lily Family (Liliaceae) ____ St. John’s Wort (Hypericum dolabriforme) ____ Nodding Wild Onion (Allium ceruum) ____ Field Garlic (A. stellatum) Parsley Family (Apiaceae) ____ Wild Asparagus (Asparagus officinalis)* ____ Queen-Anne’s Lace (Daucus carota)* ____ Orange Daylily (Hemerocallis fulva)* ____ False Solomon’s Seal Primrose Family (Primulaceae) (Smilacina acemosa) ____ Fringed Loosestrife (Lysimachia ciliata) Nettle Family (Urticaceae) Gentian Family (Gentianaceae) ____ Stinging Nettle (Urtica dioica) ____ Rose Pink (Sabatia angularis) Knotweed Family (Polygonaceae) Dogbane Family (Apocynaceae) ____ Curled Dock (Rumex crispus)* ____ Intermediate Dogbane (Apocynum medium) Pink Family (Caryophyllaceae) ____ Myrtle (Vinca minor)* ____ Deptford Pink (Dianthus armeria)* ____ Common Chickweed (Stellavia media)* Milkweed Family (Asclepiadaceae) ____ Swamp Milkweed (Asclepias incartata) Buttercup Family (Ranunculaceae) ____ Common Milkweed (A.syriaca) ____ Thimbleweed (Anemone virginiana) ____ Butterfly Weed (A. tuberosa) ____ Cliff Meadow Rue (Thalictrum clavatum) ____ Whirled Milkweed (A. verticillata) ____ Tall Meadow Rue (T. polygamum) ____ Green Milkweed (A. -

Lamiales – Synoptical Classification Vers
Lamiales – Synoptical classification vers. 2.6.2 (in prog.) Updated: 12 April, 2016 A Synoptical Classification of the Lamiales Version 2.6.2 (This is a working document) Compiled by Richard Olmstead With the help of: D. Albach, P. Beardsley, D. Bedigian, B. Bremer, P. Cantino, J. Chau, J. L. Clark, B. Drew, P. Garnock- Jones, S. Grose (Heydler), R. Harley, H.-D. Ihlenfeldt, B. Li, L. Lohmann, S. Mathews, L. McDade, K. Müller, E. Norman, N. O’Leary, B. Oxelman, J. Reveal, R. Scotland, J. Smith, D. Tank, E. Tripp, S. Wagstaff, E. Wallander, A. Weber, A. Wolfe, A. Wortley, N. Young, M. Zjhra, and many others [estimated 25 families, 1041 genera, and ca. 21,878 species in Lamiales] The goal of this project is to produce a working infraordinal classification of the Lamiales to genus with information on distribution and species richness. All recognized taxa will be clades; adherence to Linnaean ranks is optional. Synonymy is very incomplete (comprehensive synonymy is not a goal of the project, but could be incorporated). Although I anticipate producing a publishable version of this classification at a future date, my near- term goal is to produce a web-accessible version, which will be available to the public and which will be updated regularly through input from systematists familiar with taxa within the Lamiales. For further information on the project and to provide information for future versions, please contact R. Olmstead via email at [email protected], or by regular mail at: Department of Biology, Box 355325, University of Washington, Seattle WA 98195, USA. -

Bignoniaceae Adventicias En La Argentina. Primera Cita De Podranea Ricasoliana Y Nuevos Registros De Campsis Radicans
Rev. Mus. Argentino Cienc. Nat., n.s. 14(1): 15-22, 2012 ISSN 1514-5158 (impresa) ISSN 1853-0400 (en línea) Bignoniaceae adventicias en la Argentina. Primera cita de Podranea ricasoliana y nuevos registros de Campsis radicans Julio A. HURRELL1, Pablo A. CabaNILLAS2, Fernando BUET COSTANTINO3 & Gustavo DELUCCHI4 1Laboratorio de Etnobotánica y Botánica Aplicada (LEBA), Facultad de Ciencias Naturales y Museo, UNLP, Calle 64 nro. 3, 1900 La Plata, Argentina, Investigador CONICET, [email protected]. 2Cátedra de Morfología Vegetal, Facultad de Ciencias Naturales y Museo, UNLP, Paseo del Bosque s/n. 1900-La Plata, Argentina, Becario CIC Buenos Aires, [email protected]. 3Laboratorio de Etnobotánica y Botánica Aplicada (LEBA), Facultad de Ciencias Naturales y Museo, UNLP, Calle 64 nro. 3, 1900 La Plata, Argentina, [email protected]. 4División Plantas Vasculares, Facultad de Ciencias Naturales y Museo, UNLP, Paseo del Bosque s/n. 1900 La Plata, Argentina, [email protected] Abstract: Bignoniaceae adventitious in Argentina. First reference to Podranea ricasoliana, and new records of Campsis radicans. This paper includes the first reference to Podranea ricasoliana and new records of Campsis radicans (Bignoniaceae) for the adventitious Argentinean Flora. Also includes observations on the expansion mechanisms and the status into the naturalization process for P. ricasoliana, besides a key to differentiate the adventitious Bignoniaceae in Argentina. Key words: Podranea ricasoliana, Campsis radicans, Bignoniaceae, Argentina, naturalization. Resumen: Esta contribución incluye la primera cita de Podranea ricasoliana y nuevos registros de Campsis ra- dicans (Bignoniaceae) para la flora adventicia argentina. Asimismo, incluye observaciones sobre los mecanismos de expansión y la situación en el proceso de naturalización de P. -

The Linderniaceae and Gratiolaceae Are Further Lineages Distinct from the Scrophulariaceae (Lamiales)
Research Paper 1 The Linderniaceae and Gratiolaceae are further Lineages Distinct from the Scrophulariaceae (Lamiales) R. Rahmanzadeh1, K. Müller2, E. Fischer3, D. Bartels1, and T. Borsch2 1 Institut für Molekulare Physiologie und Biotechnologie der Pflanzen, Universität Bonn, Kirschallee 1, 53115 Bonn, Germany 2 Nees-Institut für Biodiversität der Pflanzen, Universität Bonn, Meckenheimer Allee 170, 53115 Bonn, Germany 3 Institut für Integrierte Naturwissenschaften ± Biologie, Universität Koblenz-Landau, Universitätsstraûe 1, 56070 Koblenz, Germany Received: July 14, 2004; Accepted: September 22, 2004 Abstract: The Lamiales are one of the largest orders of angio- Traditionally, Craterostigma, Lindernia and their relatives have sperms, with about 22000 species. The Scrophulariaceae, as been treated as members of the family Scrophulariaceae in the one of their most important families, has recently been shown order Lamiales (e.g., Takhtajan,1997). Although it is well estab- to be polyphyletic. As a consequence, this family was re-classi- lished that the Plocospermataceae and Oleaceae are their first fied and several groups of former scrophulariaceous genera branching families (Bremer et al., 2002; Hilu et al., 2003; Soltis now belong to different families, such as the Calceolariaceae, et al., 2000), little is known about the evolutionary diversifica- Plantaginaceae, or Phrymaceae. In the present study, relation- tion of most of the orders diversity. The Lamiales branching ships of the genera Craterostigma, Lindernia and its allies, hith- above the Plocospermataceae and Oleaceae are called ªcore erto classified within the Scrophulariaceae, were analyzed. Se- Lamialesº in the following text. The most recent classification quences of the chloroplast trnK intron and the matK gene by the Angiosperm Phylogeny Group (APG2, 2003) recognizes (~ 2.5 kb) were generated for representatives of all major line- 20 families. -

Available Rapid Growing Vines for the United States
ARNOLDIA A continuation of the BULLETIN OF POPULAR INFORMATION of the Arnold Arboretum, Harvard University VOLUME 4 DF.CEMBER 8, 1944 NUMBERS 9-11I AVAILABLE RAPID GROVfIN(~ VINES FOR THE UNITED STATES play a very essential part in any garden, and rapid growing vines are VINESfrequently desired for some particular purpose which no other plant material will fulfill. Sometimes they are needed only temporarily; other times they are needed permanently. Rapid growing ines are not always the most ornamental, but, since their number is rather large, some of the best will be found among them. Nor are the most ornamental vines always the easiest to obtain. Rapid growing vines that are easily obtainable are very much of interest and are in de- mand throughout the country. Consequently, this number of Arnoldia deals with those rapid growing vines, easily obtainable, that are recommended in different areas of the C’nited States. They may not all be of prime ornamental value when <·ompared with some of the rarer ones, but their rapid habit of growth makes them of considerable value for certain screening purposes. The information in this issue of Arnoldia is taken from a report prepared a short time ago when there was a great deal of interest in the camouflaging of various installations in this country, both public and private. Various horticul- turists’t mndely separated parts of the country contributed information on the * Edgar Anderson, Missouri Botanical Garden, St. Louis, Missouri W. H. Friend, Texas Agricultural Experiment Station, Weslaco, Texas Norvell Gillespie, O.C. D., San Francisco, California John Hanley, University of Washington Arboretum, Seattle, Washington A.