1 Oral Pathological Conditions in Early Postcontact Guale, St
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

View / Open Gregory Oregon 0171N 12796.Pdf
CHUNKEY, CAHOKIA, AND INDIGENOUS CONFLICT RESOLUTION by ANNE GREGORY A THESIS Presented to the Conflict and Dispute Resolution Program and the Graduate School of the University of Oregon in partial fulfillment of the requirements for the degree of Master of Science June 2020 THESIS APPROVAL PAGE Student: Anne Gregory Title: Chunkey, Cahokia, and Indigenous Conflict Resolution This thesis has been accepted and approved in partial fulfillment of the requirements for the Master of Science degree in the Conflict and Dispute Resolution Program by: Kirby Brown Chair Eric Girvan Member and Kate Mondloch Interim Vice Provost and Dean of the Graduate School Original approval signatures are on file with the University of Oregon Graduate School. Degree awarded June 2020. ii © 2020 Anne Gregory This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs (United States) License. iii THESIS ABSTRACT Anne Gregory Master of Science Conflict and Dispute Resolution June 2020 Title: Chunkey, Cahokia, and Indigenous Conflicts Resolution Chunkey, a traditional Native American sport, was a form of conflict resolution. The popular game was one of several played for millennia throughout Native North America. Indigenous communities played ball games not only for the important culture- making of sport and recreation, but also as an act of peace-building. The densely populated urban center of Cahokia, as well as its agricultural suburbs and distant trade partners, were dedicated to chunkey. Chunkey is associated with the milieu surrounding the Pax Cahokiana (1050 AD-1200 AD), an era of reduced armed conflict during the height of Mississippian civilization (1000-1500 AD). The relational framework utilized in archaeology, combined with dynamics of conflict resolution, provides a basis to explain chunkey’s cultural impact. -
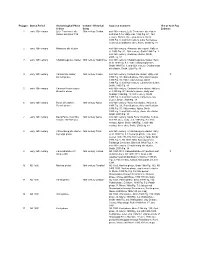
Occupation Polygons
Polygon Date & Period Archaeological Phase Cultural - Historical Source & Comment Hist or Arch Pop & Sites Group Estimate 1 early 16th century Little Tennessee site 16th century Chiaha mid-16th century, Little Tennessee site cluster cluster and sites 7-19 and sites 7-19, Hally et al. 1990:Fig. 9.1; 16th century, Chiaha, three populations, Smith 1989:Fig. 1; mid-16th century, Little Tennessee cluster plus additional sites, Smith, 2000:Fig. 18 2 early 16th century Hiwassee site cluster mid-16th century, Hiwassee site cluster, Hally et al. 1990:Fig. 9.1; 16th century, Smith 1989:Fig. 1; mid-16th century, Hiwassee cluster, Smith, 2000:Fig. 18 3 early 16th century Chattanooga site cluster 16th century Napochies mid-16th century, Chattanooga site cluster, Hally et al. 1990:Fig. 9.1; 16th century Napochies, Smith 1989:Fig. 1; mid-16th century, Chattanooga site cluster, Smith, 2000:Fig. 18 4 early 16th century Carters site cluster; 16th century Coosa mid-16th century, Carters site cluster, Hally et al. X Barnett phase 1990:Fig. 9.1; Barnett phase, Hally and Rudolph 1986:Fig. 15; 16th century Coosa, Smith 1989:Fig. 1; mid-16th century, Carters site cluster, Smith, 2000:Fig. 18 5 early 16th century Cartersville site cluster; mid-16th century, Cartersville site cluster, Hally et Brewster phase al. 1990:Fig. 9.1; Brewster phase, Hally and Rudolph 1986:Fig. 15; 16th century, Smith 1989:Fig. 1; mid-16th century, Cartersville site cluster, Smith, 2000:Fig. 18 6 early 16th century Rome site cluster; 16th century Apica mid-16th century, Rome site cluster, Hally et al. -

Mississippi Period Archaeology of the Georgia Coastal Zone
UNIVERSITY OF GEORGIA Laboratory of Archaeology Series Report No. 23 Georgia Archaeological Research Design Paper, No. 1 - MISSISSIPPI PERIOD ARCHAEOLOGY OF THE GEORGIA COASTAL ZONE II I I I \ I By Morgan R. Crook, Jr. I Department of Sociology and Anthropology West Georgia College \ Department of Anthropology I Georgia State University 1986 Reprinted, 1995 TABLE OF CONTENTS INTRODUCTION . 4 THE COASTAL ZONE ENVIRONMENT 5 THE STRUCTURAL MODEL . 11 Ethnohistoric Summary The Annual Model Discussion GEORGIA COASTAL MISSISSIPPI PERIOD: THE ARCHAEOLOGY . 34 Background Irene Mound Site Altarnaha and Savannah Regions Barrier Islands Marsh Islands Princess Ann Fonnation Interior Coastal Zone DISCUSSION AND RECOMMENDATIONS . 52 The Mississippi Period Adaptation Evaluation of the Structural Model Research Needs Management Recommendations COMMENTS . 58 Jerald T. Milanich Robin L. Smith Charles E. Pearson Stephen Wi l liams REPLY . 71 REFERENCES CITED 75 LIST OF FIGURES FIGURE 1 Major Geological Formations of the Coastal Zone . .. 8 FIGURE 2 The Guale Annual Model ....................... 18 FIGURE 3 Monthly Occurrence of Four Fish Families in Georgia Tidal Streams ...................... 23 FIGURE 4 Archaeological Locations ...................... 35 FIGURE 5 Coastal Mississippi Period C-14 Dates ............... 39 4 INTRODUCTION This document represents an effort to synthesize existing archaeological information concerning the Mississippi Period (A.D. 900-1540) in the Georgia coastal zone. Its purpose is to provide an operating plan for the protection of important cultural resources of this period on the coast. As with the other 35 operating plans being developed for Georgia's cultural resources, this one provides basic information for effective management and protection. This basic information includes synthesis and evaluation of the available archaeological information, identification of data needs, formulation of significance criteria, and development of an ideal plan for preservation and protection (see Crook 1985). -

Uga Lab Series 31.Pdf
University of Georgia Laboratory of Archaeology Series Report No. 31 Georgia Archaeological Research Design Paper No.8 mSTORIC INDIAN PERIOD ARCHAEOWGY OF THE GEORGIA COASTAL ZONE By David Hurst Thomas American Museum of Natural History March, 1993 TABLE OF CONTENTS I. OBJECTIVE ........................................................................................................ I II. STATEMENT OF PERSPECTIVE .................................................................. 2 III. THE COASTAL ZONE ENVIRONMENT .................................................... 7 IV. THE GUALE: ABORIGINAL PEOPLE OF THE GEORGIA COAST ...... 9 Patterns of Guale Subsistence Sociopolitical Organization V. HISTORY OF EUROPEAN-NATIVE AMERICAN CONTACTS ON THE GEORGIA COAST .................................................................. 12 The First European Settlement in the United States Where is San Miguel de Gualdape? Jean Ribaut First Missionaries on the Georgia Coast The Guale Rebellion of 1597 Restoration of the Franciscan Missions Spanish-British Conflicts The Dawn of Georgia's Anglo-American Period Native Americans during Georgia's Colonial Period VI. ARCHAEOLOGY OF THE HISTORIC [NDIAN PER[OD ON THE GEORGIA COAST ................................................................................... 22 The Ceramic Chronologies Mission-period Archaeology of the Georgia Coast VII. KEY RESEARCH DOMAINS ..................................................................... 40 Research Domain I. The Chronology Problem Chronlogy: Some Basic Concepts Research -

Gpb Textbook Unit 2 Final.Pdf
OUR STATE and OUR NATION UNIT Georgia Studies 2 GEORGIA EVENTS EVENTS ELSEWHERE Before it Was Georgia B.C. 12,000 People first arrive in North America CHAPTER 3 10,000 10,000 Let’s Talk History People arrive in the Paleo-Indian period begins; Southeast animals domesticated in Near Why Study History? East How to Do History The Dating Game 8000 Archaic period begins; agriculture appears in Near East CHAPTER 4 Georgia’s Prehistoric Past 4000 Unearthing Clues to Georgia’s Civilization develops in Near Prehistoric Past East; beginning of recorded history Georgia’s First Inhabitants 2500 CHAPTER 5 Egyptians build Great Pyramid Europe Discovers at Giza the New World The Age of Discovery 2000 Pottery first made in North Spain Comes to the Southeast America (near Augusta) England Comes to North America 1000 Woodland period begins A.D. Birth of Christ 1000 Mississippian period begins; Leif Ericson explores Newfoundland 1492 Columbus lands in West Indies 1498 John Cabot explores North American coast, possibly as far south as Florida 1513 Ponce de Leon becomes first European to land on North American mainland 1526 Ayllon colony briefly settles on Georgia coast 1540 De Soto expedition first to explore Georgia’s interior 1565 Spanish destroy French Fort Caroline, build St. Augustine 1566 Spanish missionaries first arrive in Guale (Georgia) 1597 Juanillo rebellion 1607 Jamestown, Virginia, becomes England’s first permanent settlement in America 1619 West Africans brought to Virginia 1663 King Charles II creates colony of Carolina 1690 Spain withdraws from Guale 1721 Britain builds Fort King George Chapter Outline Chapter 3 Why Study History? Foreword How to Do History Starting with Questions Sources of Information Using Primary Sources Analyzing and Evaluating Information Georgia Standards of Excellence The Dating Game Correlations Using a Timeline B.C. -

Indians in the Kanawha-New River Valley, 1500-1755 Isaac J
Graduate Theses, Dissertations, and Problem Reports 2015 Maopewa iati bi: Takai Tonqyayun Monyton "To abandon so beautiful a Dwelling": Indians in the Kanawha-New River Valley, 1500-1755 Isaac J. Emrick Follow this and additional works at: https://researchrepository.wvu.edu/etd Recommended Citation Emrick, Isaac J., "Maopewa iati bi: Takai Tonqyayun Monyton "To abandon so beautiful a Dwelling": Indians in the Kanawha-New River Valley, 1500-1755" (2015). Graduate Theses, Dissertations, and Problem Reports. 5543. https://researchrepository.wvu.edu/etd/5543 This Dissertation is brought to you for free and open access by The Research Repository @ WVU. It has been accepted for inclusion in Graduate Theses, Dissertations, and Problem Reports by an authorized administrator of The Research Repository @ WVU. For more information, please contact [email protected]. Maopewa iati bi: Takai Toñqyayuñ Monyton “To abandon so beautiful a Dwelling”: Indians in the Kanawha-New River Valley, 1500-1755 Isaac J. Emrick Dissertation submitted to the Eberly College of Arts and Sciences at West Virginia University in partial fulfillment of the requirements for the degree of Doctor of Philosophy in History Tyler Boulware, Ph.D., Chair Kenneth Fones-Wolf, Ph.D. Joseph Hodge, Ph.D. Michele Stephens, Ph.D. Department of History & Amy Hirshman, Ph.D. Department of Sociology and Anthropology Morgantown, West Virginia 2015 Keywords: Native Americans, Indian History, West Virginia History, Colonial North America, Diaspora, Environmental History, Archaeology Copyright 2015 Isaac J. Emrick ABSTRACT Maopewa iati bi: Takai Toñqyayuñ Monyton “To abandon so beautiful a Dwelling”: Indians in the Kanawha-New River Valley, 1500-1755 Isaac J. -

And Seventeenth-Century Native American and Hispanic Transformations of the Georgia Bight Landscapes
Bull. Fla. Mus. Nat. Hist. (2003) 44(1) 183-198 183 IMAGINING SIXTEENTH- AND SEVENTEENTH-CENTURY NATIVE AMERICAN AND HISPANIC TRANSFORMATIONS OF THE GEORGIA BIGHT LANDSCAPES Donna L. Ruhl' Various subfields of archaeology, including archaeobotany, zooarchaeology, archaepedology, and bioarchaeology, are interrelated in this essay to provide a provisional look at the interactions between humans, both Native American and European, and the environment that impacted the landscape and land use reconstruction. Focusing on the archaeobotany of both precontact sites andsixteenth- and seventeenth-century mission-period sites along coastal La Florida, Native American and Spanish use of and impact on the plants and land of the Georgia Bight reveal many transformations. Areas of the landscape were altered in part by Hispanic introductions, including architecture and agrarian practices that invoked not only a difference in degree, but, in some cases, in kind that persist to the present. These changes, however, had far less impact than subsequent European introductions would bring to this region of the.Georgia Bight and its adjacent area. Key words: archaeobotany, Georgia Bight, landscape archaeology, Native American settlement, Spanish settlement Reconstructing landscapes from the archaeological the emergent field of environmental archaeology can record is complex because landscape transformations offer, I selected the lower Atlantic coast for two reasons. minimally entail intentional constructs of buildings and First, much zooarchaeological research has been land use (gardens, fields, roads, pathways, tree plantings, generated in Elizabeth S. Wing's laboratory from this and the like), incidental and accidental human region. Second, for more than a decade archaeobotanical manipulation, and such natural phenomena as floods, research has been encouraged by and has benefited from droughts, and high winds (Ashton 1985; Beaudry 1986; Dr. -

Balancing Cross-Cultural Communication in the Pre-Colonial and Colonial Southeast
University of North Florida UNF Digital Commons UNF Graduate Theses and Dissertations Student Scholarship 2014 From Chaos to Order: Balancing Cross-Cultural Communication in the Pre-Colonial and Colonial Southeast Nicole Lynn Gallucci University of North Florida, [email protected] Follow this and additional works at: https://digitalcommons.unf.edu/etd Part of the Cultural History Commons, Social History Commons, and the United States History Commons Suggested Citation Gallucci, Nicole Lynn, "From Chaos to Order: Balancing Cross-Cultural Communication in the Pre-Colonial and Colonial Southeast" (2014). UNF Graduate Theses and Dissertations. 516. https://digitalcommons.unf.edu/etd/516 This Master's Thesis is brought to you for free and open access by the Student Scholarship at UNF Digital Commons. It has been accepted for inclusion in UNF Graduate Theses and Dissertations by an authorized administrator of UNF Digital Commons. For more information, please contact Digital Projects. © 2014 All Rights Reserved FROM CHAOS TO ORDER: BALANCING CROSS-CULTURAL COMMUNICATION IN THE PRE-COLONIAL AND COLONIAL SOUTHEAST By Nicole Gallucci A thesis submitted to the Department of History in partial fulfillment of the requirements for the degree of Master of Arts in History UNIVERSITY OF NORTH FLORIDA COLLEGE OF ARTS AND SCIENCES June, 2014 Unpublished work © Nicole Gallucci Certificate of Approval The Thesis of Nicole Gallucci is approved: _______________________________________ Date: __________________ Dr. Denise Bossy _______________________________________ Date: __________________ Dr. Denice Fett _______________________________________ Date: __________________ Dr. Keith Ashley Accepted for the History Department: _______________________________________ Date: __________________ Dr. Charles E. Closmann Chair Accepted for the College of Arts and Sciences: _______________________________________ Date: __________________ Dr. -

Yamassee Origins and the Development of the Carolina-Florida Frontier
Yamassee Origins and the Development of the Carolina-Florida Frontier John E. Worth The Coosawattee Foundation Essay prepared for the fifth annual conference of the Omohundro Institute of Early American History and Culture, June 12, 1999, Austin, Texas. The Yamassee Indians have long figured prominently in historical accounts of the early history of the Southern colonies. Their short-lived tenure in the late 17th-century missions of Spanish Florida, and along the southern frontier of early 18th-century Carolina, ensured them a notable place in Southern history alongside other contemporaneous groups such as the Creek and Cherokee, despite their eventual exile and virtual extinction before the time of the American Revolution. In 1715, however, the Yamassee took center stage as they sparked a widespread and violent revolt that left hundreds of South Carolina settlers and traders dead, and that ultimately reconfigured the entire social landscape of the southeastern borderlands. Nevertheless, despite their relatively prominent historical visibility, the Yamassees have always remained something of an enigma for historians, anthropologists, and others searching for clues as to their origins. In recent years, substantial advances have been made in the archaeology and early colonial ethnohistory of the Southeastern Indians, and particularly regarding the many groups that inhabited the broad region that has been called the Spanish Borderlands, generally positioned between Spanish Florida and the southernmost English colonies of Carolina and Georgia. As a result of these advances, many of which are still ongoing, present-day scholars are now able to draw upon a far more complete and detailed database of archaeological and particularly documentary data than has ever been available before. -

Engendered Death: a Comprehensive Analysis of Identity in The
ENGENDERED DEATH: A COMPREHENSIVE ANALYSIS OF IDENTITY IN THE MISSION SYSTEM OF 17TH CENTURY SPANISH FLORIDA by Katherine Louise Brewer B.S., Portland State University, 2010 A thesis submitted to the Department of Anthropology College of Arts, Social Sciences, and Humanities The University of West Florida In partial fulfillment of the requirements for the degree of Master of Arts 2014 © 2014 Katherine Louise Brewer The thesis of Katherine Louise Brewer is approved: ____________________________________________ ________________ Amy Mitchell-Cook, Ph.D., Committee Member Date ____________________________________________ ________________ Ramie A. Gougeon, Ph.D., Committee Member Date ____________________________________________ ________________ John E. Worth, Ph.D., Committee Chair Date Accepted for the Department/Division: ____________________________________________ ________________ John R. Bratten, Ph.D., Chair Date Accepted for the University: ____________________________________________ ________________ Richard S. Podemski, Ph.D., Dean of Graduate Studies Date ACKNOWLEDGMENTS I would first like to thank my thesis committee, Dr. John Worth, Dr. Ramie Gougeon, and Dr. Amy Mitchell-Cook for all of the help and insight they have given me. It has been invaluable. I would also like to thank Dr. Elizabeth Benchley, Jan Lloyd, and the Archaeology Institute for giving me the financial resources I needed as well as a place of employment in the field that I love while I completed this program. It has been a wonderful opportunity to gain the experience that I have both in the lab and in the field. I have to thank all the friends and fellow graduate students who have helped me make it through this whole process. Special thanks go to Michelle Pigott, Salina Hebert, Dani Mount, and Jennifer Walborn for all of the words of encouragement and all of the support you have given me when I have needed it. -

The Rise of the Indigenous Slave Trade and Diaspora from Española to the Circum-Caribbean, 1492-1542
Indian Harvest: The Rise of the Indigenous Slave Trade and Diaspora from Española to the Circum-Caribbean, 1492-1542 By Erin Woodruff Stone Dissertation Submitted to the Faculty of the Graduate School of Vanderbilt University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in History May, 2014 Nashville, Tennessee Approved: Jane G. Landers, Ph.D. Edward Wright-Rios, Ph.D. Dan Usner, Ph.D. Steven Wernke, Ph.D. Copyright © 2014 by ErinWoodruff Stone All Rights Reserved Acknowlegdements This work would not have been possible without financial support from Vanderbilt, particulary the History Department, Graduate School, and Latin American Studies Program. I am also greatly indebted to the Institute of Internal Education, the Andrew W. Mellon Foundation, Harvard University’s Atlantic History Seminar, and the University of Minnesota’s Program for Cultural Cooperation. I am grateful to all those I have worked with along the way who offered advice, criticism, guidance, and intellectual support. I would especially like to thank my advisor Dr. Jane Landers. She taught me invaluable personal and profession lessons, provided me with endless hours of her time, and never failed to support me. I also want to thank the rest of my committee; Dr. Edward Wright-Rios, Dr. Steven Wernke, and Dr. Dan Usner, all of whom contributed to the shape of the project and offered great, if often hard to hear criticism, from the dissertation’s inception to its completion. Outside of Vanderbilt I need to thank both Dr. Ida Altman and Dr. J. Michael Francis, both of whom read early versions of chapters, supported me at conferences, and gave me archival leads. -

Exploring Mission Life in 18Th-Century West Florida: 2011 Excavations at San Joseph De Escambe John Worth, Norma J. Harris, Jenn
Exploring Mission Life in 18th-Century West Florida: 2011 Excavations at San Joseph de Escambe John Worth, Norma J. Harris, Jennifer Melcher, Danielle Dadiego University of West Florida Abstract In 2011, University of West Florida terrestrial field school students participated in a third consecutive year of excavations at Mission San Joseph de Escambe, located north of modern Pensacola between 1741 and 1761. Inhabited by Apalachee Indians and a small number of Franciscan friars and married Spanish soldiers, as well as a Spanish cavalry garrison late in the mission's history, the site's pristine archaeological deposits are gradually revealing details about mission life along this northernmost frontier of 18th-century West Florida. Ongoing block excavations have continued to expose a complex assemblage of architectural features separated by both vertical and horizontal stratigraphy, including several overlapping wall-trench structures capped with what seems to be a clay floor, and a large structure believed to be the cavalry barracks. Artifacts ranging from a predominantly Apalachee ceramic assemblage to an assortment of European trade goods continue to refine our understanding of this important site. Paper presented at the 2012 Conference of the Society for Historical Archaeology, Baltimore, Maryland, January 6, 2012. 1 Colonial mission communities have long been recognized by historians and anthropologists as crucibles of dynamic cultural transformation. Missions were not simply the setting for religious conversion, but in fact represented the primary stage on which two cultures—indigenous and colonizing—confronted each other in the context of daily life over the course of many years, decades, and even generations. Out of this long-term process of missionization were forged new cultural identities that were not simply the replacement of the indigenous culture by the colonizing culture, or some piecemeal blend of the two, but which instead manifested new cultural formations that have been variously described as creole or hybrid cultures.