Development and Validation of Analytical Methods for the Determination of Some Skeletal Muscle Relaxant Drugs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

Use of Chemical Chelators As Reversal Agents for Drug
(19) TZZ_ _ZZZ_T (11) EP 1 210 090 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 31/724 (2006.01) A61K 31/194 (2006.01) 18.06.2014 Bulletin 2014/25 A61P 39/04 (2006.01) (21) Application number: 00964006.1 (86) International application number: PCT/EP2000/007694 (22) Date of filing: 07.08.2000 (87) International publication number: WO 2001/012202 (22.02.2001 Gazette 2001/08) (54) USE OF CHEMICAL CHELATORS AS REVERSAL AGENTS FOR DRUG- INDUCED NEUROMUSCULAR BLOCK VERWENDUNG VON CHEMISCHEN CHELATOREN ZUR UMKEHRUNG VON PHARMAKOLOGISCH-INDUZIERTER NEUROMUSKULÄRER BLOCKIERUNG UTILISATION D’AGENTS CHIMIQUES CHELATANTS COMME AGENTS DE NEUTRALISATION DU BLOCAGE NEUROMUSCULAIRE PROVOQUE PAR DES MEDICAMENTS (84) Designated Contracting States: (56) References cited: AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU AU-A- 3 662 895 MC NL PT SE • B DESIRE: "Inactivaton of sarin and soman by (30) Priority: 13.08.1999 EP 99306411 cyclodextrins in vitro" EXPERIENTIA, vol. 43, no. 4, 1987, pages 395-397, XP000907287 (43) Date of publication of application: • B. DESIRE: "Interaction of soman with beta- 05.06.2002 Bulletin 2002/23 cyclodextrin" FUNDAMENTAL AND APPLIED TOXICOLOGY, vol. 7, no. 4, 1986, pages 647-657, (73) Proprietor: Merck Sharp & Dohme B.V. XP000911170 2031 BN Haarlem (NL) • C. MAY: "Development of a toxin-binding agent as a treatment for tunicamycinuracil toxicity: (72) Inventors: protection against tunicamycin poisoning of • BOM, Antonius, Helena, Adolf sheep" AUSTRALIAN VETERINARY JOURNAL, Ratho, Midlothian EH28 8NY (GB) vol. 76, no. -

(12) Patent Application Publication (10) Pub. No.: US 2004/0224012 A1 Suvanprakorn Et Al
US 2004O224012A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0224012 A1 Suvanprakorn et al. (43) Pub. Date: Nov. 11, 2004 (54) TOPICAL APPLICATION AND METHODS Related U.S. Application Data FOR ADMINISTRATION OF ACTIVE AGENTS USING LIPOSOME MACRO-BEADS (63) Continuation-in-part of application No. 10/264,205, filed on Oct. 3, 2002. (76) Inventors: Pichit Suvanprakorn, Bangkok (TH); (60) Provisional application No. 60/327,643, filed on Oct. Tanusin Ploysangam, Bangkok (TH); 5, 2001. Lerson Tanasugarn, Bangkok (TH); Suwalee Chandrkrachang, Bangkok Publication Classification (TH); Nardo Zaias, Miami Beach, FL (US) (51) Int. CI.7. A61K 9/127; A61K 9/14 (52) U.S. Cl. ............................................ 424/450; 424/489 Correspondence Address: (57) ABSTRACT Eric G. Masamori 6520 Ridgewood Drive A topical application and methods for administration of Castro Valley, CA 94.552 (US) active agents encapsulated within non-permeable macro beads to enable a wider range of delivery vehicles, to provide longer product shelf-life, to allow multiple active (21) Appl. No.: 10/864,149 agents within the composition, to allow the controlled use of the active agents, to provide protected and designable release features and to provide visual inspection for damage (22) Filed: Jun. 9, 2004 and inconsistency. US 2004/0224012 A1 Nov. 11, 2004 TOPCAL APPLICATION AND METHODS FOR 0006 Various limitations on the shelf-life and use of ADMINISTRATION OF ACTIVE AGENTS USING liposome compounds exist due to the relatively fragile LPOSOME MACRO-BEADS nature of liposomes. Major problems encountered during liposome drug Storage in vesicular Suspension are the chemi CROSS REFERENCE TO OTHER cal alterations of the lipoSome compounds, Such as phos APPLICATIONS pholipids, cholesterols, ceramides, leading to potentially toxic degradation of the products, leakage of the drug from 0001) This application claims the benefit of U.S. -

Download Document
FAM001159-0001 intervals of 4-7 days to usual dose of 75-100 mg Dolmatil® (Sanofi-Synthelabo) ~ 100 rag/5 mL, thioridazine 100 rag/ per course and max. 4 injections; max. duration of daily according to response; CHILD not recom- Tablets, both scored, sulpiride 200 rag, net price ’ net price 300 mL = £7.14. Label: 2 treatment 2 weeks---if maintenance treatment mended 100-tabpack=£13.85;400mg(f/c), 100-tab ! fNote. These suspensions should not be diluted but the necessary change to an oral antipsychotic 2-3 Short-term adjunctive management of severe pack = £36¯29. Label: 2 .: :~t~a preparations may be mixed with each other to days after last injection, or to a longer acting anti- anxiety, 15-20rag daily in divided doses; max. Sulpltil® (Pharmacia) I~ ~0iovid¢ intermediate strengths psychotic depot injection given concomitantly 40 mg daily; CHILD not recommended Tablets, scored, sulpiride 200 rag. Net price 28-tab "ff~-~p, brown, thioridazine (as hydrochloride) with last injection of zuclopenthixol acetate; By deep intramuscular injection, psychoses, mania, pack = £4.29; 112-tab pack = £12.85. Label: 2 ~ff.~5"~mg/5 mL, net price 300 mL = £1.98. Label: 2 CHILD not recommended prochlorperazine mesilate 12.5-25 mg 2-3 times Sulpor® (Rosemont) IPoMI Clopi~ol Acuphase® (Lundbeck) daily; CHILD not recommended Oral solution, sugar-free, lemon- and aniseed-fla. LUOPERAZINE Injection (oily), zuclopenthixol acetate 50 mg/mL. By rectum in suppositories, psychoses, mania, the voured, sulpiride 200 mg/5 mL, net price 150 mL (’~’n~ications: see under Dose; anti-emetic (section Net price I-mL amp = £5.20; 2-mL amp = £10.03 equivalent of prochlorperazine maleate 25 mg 2- = £27.00. -

University Microfilms
INFORMATION TO USERS This dissertation was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. You w ill find a good image of the page in the adjacent frame. 3. When a map, drawing or chart, etc., was part of the material being photographed the photographer followed a definite method in "sectioning" the material. It is customary to begin photoing at the upper left hand corner of a large sheet and to continue photoing from left to right in equal sections with a small overlap. If necessary, sectioning is continued again — beginning below the first row and continuing on until complete. 4. The majority of users indicate that the textual content is of greatest value, however, a somewhat higher quality reproduction could be made from "photographs" if essential to the understanding of the dissertation. -

PMBJP Product.Pdf
Sr. Drug Generic Name of the Medicine Unit Size MRP Therapeutic Category No. Code Analgesic & Antipyretic / Muscle 1 1 Aceclofenac 100mg and Paracetamol 325 mg Tablet 10's 10's 8.00 relaxants Analgesic & Antipyretic / Muscle 2 2 Aceclofenac Tablets IP 100mg 10's 10's 4.37 relaxants Acetaminophen 325 + Tramadol Hydrochloride 37.5 film Analgesic & Antipyretic / Muscle 3 4 10's 8.00 coated Tablet 10's relaxants Analgesic & Antipyretic / Muscle 4 5 ASPIRIN Tablets IP 150 mg 14's 14's 2.70 relaxants DICLOFENAC 50 mg+ PARACETAMOL 325 mg+ Analgesic & Antipyretic / Muscle 5 6 10's 11.30 CHLORZOXAZONE 500 mg Tablets 10's relaxants Diclofenac Sodium 50mg + Serratiopeptidase 10mg Tablet Analgesic & Antipyretic / Muscle 6 8 10's 12.00 10's relaxants Analgesic & Antipyretic / Muscle 7 9 Diclofenac Sodium (SR) 100 mg Tablet 10's 10's 6.12 relaxants Analgesic & Antipyretic / Muscle 8 10 Diclofenac Sodium 25mg per ml Inj. IP 3 ml 3 ml 2.00 relaxants Analgesic & Antipyretic / Muscle 9 11 Diclofenac Sodium 50 mg Tablet 10's 10's 2.90 relaxants Analgesic & Antipyretic / Muscle 10 12 Etoricoxilb Tablets IP 120mg 10's 10's 33.00 relaxants Analgesic & Antipyretic / Muscle 11 13 Etoricoxilb Tablets IP 90mg 10's 10's 25.00 relaxants Analgesic & Antipyretic / Muscle 12 14 Ibuprofen 400 mg + Paracetamol 325 mg Tablet 10's 15's 5.50 relaxants Analgesic & Antipyretic / Muscle 13 15 Ibuprofen 200 mg film coated Tablet 10's 10's 1.80 relaxants Analgesic & Antipyretic / Muscle 14 16 Ibuprofen 400 mg film coated Tablet 10's 15's 3.50 relaxants Analgesic & Antipyretic -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Assessment of Molecular Action of Direct Gating and Allosteric Modulatory Effects of Carisoprodol (Somartm) on GABA a Receptors
Graduate Theses, Dissertations, and Problem Reports 2015 Assessment of molecular action of direct gating and allosteric modulatory effects of carisoprodol (SomaRTM) on GABA A receptors Manoj Kumar Follow this and additional works at: https://researchrepository.wvu.edu/etd Recommended Citation Kumar, Manoj, "Assessment of molecular action of direct gating and allosteric modulatory effects of carisoprodol (SomaRTM) on GABA A receptors" (2015). Graduate Theses, Dissertations, and Problem Reports. 6022. https://researchrepository.wvu.edu/etd/6022 This Dissertation is protected by copyright and/or related rights. It has been brought to you by the The Research Repository @ WVU with permission from the rights-holder(s). You are free to use this Dissertation in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you must obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This Dissertation has been accepted for inclusion in WVU Graduate Theses, Dissertations, and Problem Reports collection by an authorized administrator of The Research Repository @ WVU. For more information, please contact [email protected]. ASSESSMENT OF MOLECULAR ACTION OF DIRECT GATING AND ALLOSTERIC MODULATORY EFFECTS OF MEPROBAMATE (MILTOWN®) ON GABAA RECEPTORS Manish Kumar, MD, MS Dissertation submitted to the School of Pharmacy at West Virginia University in partial fulfillment of Requirements -

10 Mg/Ml Solution for Injection/Infusion Atracurium
Package leaflet: Information for the patient <Product name> 10 mg/ml solution for injection/infusion Atracurium besilate Read all of this leaflet carefully before you start using this medicine because it contains important information for you. - Keep this leaflet. You may need to read it again. - If you have any further questions, ask your doctor or nurse. - If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet 1. What <Product name> is and what it is used for 2. What you need to know before you receive <Product name> 3. How to use <Product name> 4. Possible side effects 5. How to store <Product name> 6. Contents of the pack and other information 1. What <Product name> is and what it is used for <Product name> belongs to a group of medicines called muscle relaxants. <Product name> is used during surgery to relax muscles and to assist with inserting a breathing tube and with artificial breathing. It is also used to help with artificial breathing at patients in intensive care units. 2. What you need to know before you receive <Product name> <Product name> cannot be administered: - if you are allergic to atracurium besilate, cisatracurium or any of the other ingredients of this medicine (listed in section 6). If you think that this applies to you talk to your doctor before <Product name> is administered to you. Warnings and precautions Talk to your doctor or nurse before using <Product name>: - if you have an allergy or bronchial -
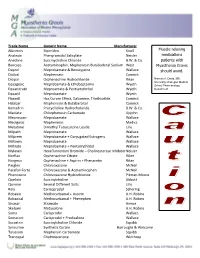
Medication Alert
Trade Name Generic Name Manufacturer Akineton Biperiden Knoll Muscle relaxing Analexin Phenyramidol Salicylate Neisler medications Anectine Succinycholine Chloride B.W. & Co. patients with Bancaps Acetaminophin, Mephenesin Butabarbital Sodium West Myasthenia Gravis Deprol Meprobamate & Benscyyzine Wallace should avoid. Dioloxl Mephensein Camrick Disipal Orphenadrine Hydrochloride Riker Norman A. David, MD, University of Oregon Medical Equagesic Meprobamate & Ethobeptazine Wyeth School Pharmacology Equanitrate Meproamate & Pentserythritol Wyeth Department Equanil Meprobamate Wyeth Flaxedil Has Curare Effect, Galiamine, Triethiodide Camrick Halabar Mephenesin & Butabarbital Camrick Kemadrin Procycllidine Hydrocholoride B.W. & Co. Maolate Chlorphenesin Carbamate Upjohn Meprospan Meprobamate Wallace Medigesic Mephenesin Medics Metubine Dimethyl Tubocurzine Loside Lilly Milpath Meprobamate Wallace Milprem Meprobamate + Conjugated Estrogens Wallace Miltown Meprobamate Wallace Miltrate Meprobamate + Pentserythritol Wallace Mylaxen Hexafluroenium Bromide – Cholinesterase Inhibitor Neisler Norflax Orphenadrine Citrate Riker Norgesic Orphenadrine + Aspirin + Phenacetin Riker Parglex Chlorzoxazone McNeil Parafon Forte Chlorzoxazone & Acetaminophen McNeil Phenoxene Chlorzoxazone Hydrochloride Pitman-Moore Quelicin Succinylcholine Abbott Quinine Several Different Salts Lilly Rela Carisoprodol Schering Robaxin Methocarbamal + Aspirin A.H. Robins Robaxisal Methocarbamal + Phenephen A.H. Robins Sinaxar Stramate Armor Skelaxin Metaxalone A.H. Robins Soma -

(12) United States Patent (10) Patent N0.: US 7,265,099 B1 Born Et A1
US007265099B1 (12) United States Patent (10) Patent N0.: US 7,265,099 B1 Born et a1. (45) Date of Patent: *Sep. 4, 2007 (54) USE OF CHEMICAL CHELATORS AS Tarver, G. et al “2-O-Substituted cyclodextrins as reversal REVERSAL AGENTS FOR DRUG-INDUCED agents . ” Bioorg. Med. Chem. (2002) vol. 10, pp 1819-1827.* NEUROMUSCULAR BLOCK Zhang, M. “Drug-speci?c cyclodextrins . ” Drugs of the Future (2003) vol. 28, no 4, pp 347-354.* (75) Inventors: Antonius Helena Adolf Bom, Lee, C. “Structure, conformation, and action of neuromuscular Midlothian (GB); Alan William Muir, blocking drugs” Brit. J. Anesth. (2001) vol. 87, no 5, pp 755-769.* Lanark (GB); David Rees, Gothenburg B Desire: “Inactivation of sarin and soman by cyclodextrins in (SE) vitro” EXPERIENTIA, vol. 43, No. 4, 1987, pp. 395-397. B. Desire: “Interaction of soman with beta-cyclodextrin” Funda (73) Assignee: Organon N.V., Oss (NL) mental and Applied Toxicology, vol. 7, No. 4, 1986, pp. 647-657. ( * ) Notice: Subject to any disclaimer, the term of this C. May: “Development of a toxin-bindng agent as a treatment for patent is extended or adjusted under 35 tunicamycinuracil toxicity: protection against tunicamycin poison U.S.C. 154(b) by 0 days. ing of sheep” Australian Veterinary Journal, vol. 76, No. 11, 1998 pp. 752-756. This patent is subject to a terminal dis K. Uekama: “Effects of cyclodextrins on chlorpromaZine-induced claimer. haemolysis and nervous systems responses” J. Pharm. Pharmacol., vol. 33, No. 11, 1981, pp. 707-710. (21) Appl. No.: 10/049,393 T. Irie: “Protective mechanism of beta-cyclodextrin for the hemolysis induced With phenothiazine neuroleptics in vitro” J. -

Carisoprodol (CAS No
National Toxicology Program Toxicity Report Series Number 56 NTP Technical Report on the Toxicity Studies of Carisoprodol (CAS No. 78-44-4) Administered by Gavage to F344/N Rats and B6C3F1 Mice August 2000 U.S. Department of Health and Human Services Public Health Service National Institutes of Health FOREWORD The National Toxicology Program (NTP) is made up of four charter agencies of the U.S. Department of Health and Human Services (DHHS): the National Cancer Institute (NCI), National Institutes of Health; the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health; the National Center for Toxicological Research (NCTR), Food and Drug Administration; and the National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention. In July 1981, the Carcinogenesis Bioassay Testing Program, NCI, was transferred to the NIEHS. The NTP coordinates the relevant programs, staff, and resources from these Public Health Service agencies relating to basic and applied research and to biological assay development and validation. The NTP develops, evaluates, and disseminates scientific information about potentially toxic and hazardous chemicals. This knowledge is used for protecting the health of the American people and for the primary prevention of disease. The studies described in this Toxicity Study Report were performed under the direction of the NIEHS and were conducted in compliance with NTP laboratory health and safety requirements and must meet or exceed all applicable federal, state, and local health and safety regulations. Animal care and use were in accordance with the Public Health Service Policy on Humane Care and Use of Animals. These studies are designed and conducted to characterize and evaluate the toxicologic potential of selected chemicals in laboratory animals (usually two species, rats and mice).