Susceptibility Testing of Yeasts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Topical and Systemic Antifungal Therapy for Chronic Rhinosinusitis (Protocol)
CORE Metadata, citation and similar papers at core.ac.uk Provided by University of East Anglia digital repository Cochrane Database of Systematic Reviews Topical and systemic antifungal therapy for chronic rhinosinusitis (Protocol) Head K, Sacks PL, Chong LY, Hopkins C, Philpott C Head K, Sacks PL, Chong LY, Hopkins C, Philpott C. Topical and systemic antifungal therapy for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 11. Art. No.: CD012453. DOI: 10.1002/14651858.CD012453. www.cochranelibrary.com Topical and systemic antifungal therapy for chronic rhinosinusitis (Protocol) Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 BACKGROUND .................................... 1 OBJECTIVES ..................................... 3 METHODS ...................................... 3 ACKNOWLEDGEMENTS . 8 REFERENCES ..................................... 9 APPENDICES ..................................... 10 CONTRIBUTIONSOFAUTHORS . 25 DECLARATIONSOFINTEREST . 26 SOURCESOFSUPPORT . 26 NOTES........................................ 26 Topical and systemic antifungal therapy for chronic rhinosinusitis (Protocol) i Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. [Intervention Protocol] Topical and systemic antifungal therapy for chronic rhinosinusitis Karen Head1, Peta-Lee Sacks2, Lee Yee Chong1, Claire Hopkins3, Carl Philpott4 1UK Cochrane Centre, -

Download Product Insert (PDF)
PRODUCT INFORMATION Isoconazole (nitrate) Item No. 30100 CAS Registry No.: 24168-96-5 Cl Formal Name: 1-[2-(2,4-dichlorophenyl)-2-[(2,6- dichlorophenyl)methoxy]ethyl]-1H- imidazole, mononitrate Synonyms: Adestan G 100, R 15454 Cl Cl MF: C18H14Cl4N2O • HNO3 FW: 479.1 N N Purity: ≥98% O Supplied as: A solid Storage: -20°C • HNO3 Cl Stability: ≥2 years Information represents the product specifications. Batch specific analytical results are provided on each certificate of analysis. Laboratory Procedures Isoconazole (nitrate) is supplied as a solid. A stock solution may be made by dissolving the isoconazole (nitrate) in the solvent of choice, which should be purged with an inert gas. Isoconazole (nitrate) is soluble in organic solvents such as DMSO and dimethyl formamide (DMF). The solubility of isoconazole (nitrate) in these solvents is approximately 10 mg/ml. Isoconazole (nitrate) is sparingly soluble in aqueous buffers. For maximum solubility in aqueous buffers, isoconazole (nitrate) should first be dissolved in DMF and then diluted with the aqueous buffer of choice. Isoconazole (nitrate) has a solubility of approximately 0.2 mg/ml in a 1:4 solution of DMF:PBS (pH 7.2) using this method. We do not recommend storing the aqueous solution for more than one day. Description Isoconazole is an imidazole with antimicrobial activity.1 It is active against clinical isolates of Candida species, including C. albicans, C. parapsilosis, C. tropicalis, C. krusei, and C. guilliermondii with MIC values ranging from 0.12 to 2 μg/ml. It is also active against the fungi T. mentagrophytes and T. rubrum when used at a concentration of 0.1 µg/ml and the bacteria C. -

Dermatologic Medication in Pregnancy
Marušić et al. Acta Dermatovenerol Croat Subcutaneous dirofilariasis Acta Dermatovenerol Croat 2009;17(1):40-47 REVIEW Dermatologic Medication in Pregnancy Petra Turčić1, Zrinka Bukvić Mokos2, Ružica Jurakić Tončić2, Vladimir Blagaić3, Jasna Lipozenčić2 1School of Pharmacy and Biochemistry, University of Zagreb; 2University Department of Dermatology and Venereology, Zagreb University Hospital Center and School of Medicine; 3University Department of Obstetrics and Gynecology, Sveti Duh General Hospital, Zagreb, Croatia Corresponding author: SUMMARY In female body, a vast number of skin changes occur Petra Turčić, Phar. M. during pregnancy. Some of them are quite distressing to many women. Department of Pharmacology Therefore, performing treatment for physiologic skin changes during pregnancy with antiinfective agents, glucocorticosteroids, topical School of Pharmacy and Biochemistry immunomodulators, retinoids, minoxidil, etc., is discussed. Drug University of Zagreb administration during pregnancy must be reasonable. Domagojeva 2 KEY WORDS: dermatologic medication, pregnancy, physiologic skin HR-10000 Zagreb changes, treatment Croatia [email protected] Received: September 1, 2008 Accepted: January 9, 2009 INTRODUCTION In female body, a vast number of changes oc- bolic imbalances (3), diabetes and cardiovascular cur during pregnancy. Some of them are quite diseases (4). Pregnancy extends and alters the distressing to many women. Therefore, perform- impact of sex differences on absorption, distribu- ing treatment for these changes during pregnancy tion, metabolism and elimination (5). Cardiac out- is discussed. Normal pregnancy needs to avoid put is elevated early and remains elevated for the harmful drugs, both prescribed and over-the coun- remainder of pregnancy. Regional blood flow can ter, and drugs of abuse, including cigarettes, alco- change, with some areas of the skin having sub- hol as well as occupational and environmental ex- stantial increases in blood flow during the course posure to potentially harmful chemicals. -

Abstract #15 Drug Repurposing for Human Visceral Leishmaniasis: Screening Marketed Antifungal Azoles for Inhibition of Leishmani
Abstract #15 Drug Repurposing for Human Visceral Leishmaniasis: Screening Marketed Antifungal Azoles for Inhibition of Leishmanial CYP5122A1 and CYP51 Enzymes Yiru Jin1, Mei Feng1, Michael Zhuo Wang1 1Department of Pharmaceutical Chemistry, University of Kansas, Lawrence, KS, USA CYP5122A1 is a novel cytochrome P450 (CYP) enzyme that is essential for the survival of Leishmania donovani, a major causative agent for human visceral leishmaniasis. Recent studies suggest that CYP5122A1 plays an important role as sterol 4-demethylase in ergosterol biosynthesis by Leishmania. Thus, CYP5122A1 inhibitors may represent a new therapeutic approach against the devastating infectious disease. To evaluate the effects of antifungal azoles on CYP5122A1, a panel of twenty marketed antifungal azoles (bifonazole, butoconazole, clotrimazole, econazole, efinaconazole, fenticonazole, fluconazole, isavuconazole, isoconazole, itraconazole, ketoconazole, miconazole, oxiconazole, posaconazole, ravuconazole, sertaconazole, sulconazole, terconazole, tioconazole, voriconazole) were screened for inhibition of CYP5122A1, as well as the lanosterol 14α-demethylase CYP51, using a fluorescence-based inhibition assay. All twenty azoles were potent inhibitors of leishmanial CYP51 with IC50 ranging from 0.031 to 0.084 M, whereas their inhibitory potencies against CYP5122A1 were much lower (0.25 to 12 M). Ravuconazole was identified as a selective CYP51 inhibitor with an IC50 value of 0.048 M and selectivity index of 250 against CYP5122A1. Interestingly, the imidazole class of antifungal azoles showed stronger inhibition against CYP5122A1 compared with the triazole class. These results can provide insights toward rational drug design of CYP5122A1 inhibitors. Future studies will attempt to obtain X-ray crystal structures of several select protein-ligand complexes and confirm the proposed biochemical roles of CYP5122A1 and CYP51 by treating parasites with selective inhibitors, followed by HPLC-MS/MS-based sterol analysis. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

Australian Statistics on Medicines 1997 Commonwealth Department of Health and Family Services
Australian Statistics on Medicines 1997 Commonwealth Department of Health and Family Services Australian Statistics on Medicines 1997 i © Commonwealth of Australia 1998 ISBN 0 642 36772 8 This work is copyright. Apart from any use as permitted under the Copyright Act 1968, no part may be repoduced by any process without written permission from AusInfo. Requests and enquiries concerning reproduction and rights should be directed to the Manager, Legislative Services, AusInfo, GPO Box 1920, Canberra, ACT 2601. Publication approval number 2446 ii FOREWORD The Australian Statistics on Medicines (ASM) is an annual publication produced by the Drug Utilisation Sub-Committee (DUSC) of the Pharmaceutical Benefits Advisory Committee. Comprehensive drug utilisation data are required for a number of purposes including pharmacosurveillance and the targeting and evaluation of quality use of medicines initiatives. It is also needed by regulatory and financing authorities and by the Pharmaceutical Industry. A major aim of the ASM has been to put comprehensive and valid statistics on the Australian use of medicines in the public domain to allow access by all interested parties. Publication of the Australian data facilitates international comparisons of drug utilisation profiles, and encourages international collaboration on drug utilisation research particularly in relation to enhancing the quality use of medicines and health outcomes. The data available in the ASM represent estimates of the aggregate community use (non public hospital) of prescription medicines in Australia. In 1997 the estimated number of prescriptions dispensed through community pharmacies was 179 million prescriptions, a level of increase over 1996 of only 0.4% which was less than the increase in population (1.2%). -
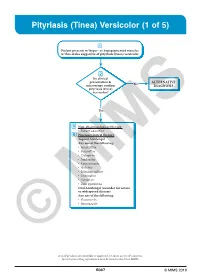
Pityriasis (Tinea) Versicolor (1 of 5)
Pityriasis (Tinea) Versicolor (1 of 5) 1 Patient presents w/ hyper- or hypopigmented macules w/ fine scales suggestive of pityriasis (tinea) versicolor 2 Do clinical presentation & No ALTERNATIVE microscopy confirm DIAGNOSIS pityriasis (tinea) versicolor? Yes A Non-pharmacological therapy • Patient education B Pharmacological therapy Topical Antifungal Any one of the following: • Amorolfi ne • Butenafi ne • Ciclopirox • Imidazoles • Ketoconazole • Naftifi ne • Selenium sulfi de • Terbinafi ne • Tolnaftate • Zinc pyrithione Oral Antifungal (consider for severe or widespread disease) Any one of the following: • Fluconazole • Itraconazole © MIMS Not all products are available or approved for above use in all countries. Specifi c prescribing information may be found in the latest MIMS. B307 © MIMS 2019 Pityriasis (Tinea) Versicolor (2 of 5) 1 EVALUATION Pityriasis (tinea) versicolor is a common, benign, superfi cial fungal infection localized to the stratum corneum • Caused by lipophilic yeasts, Malassezia species, part of the normal fl ora of the human skin Clinical Presentation • May present as chronic or recurrent infection & may occur in healthy individuals - More common in summer than winter months • Predominates in young adults when the sebaceous glands are most active • Presents w/ multiple well-demarcated macules or patches & fi nely scaled plaques w/ hypopigmentation or hyperpigmentation, hence the term “versicolor” • Tends to be asymptomatic & is mainly a cosmetic concern but pruritus may or may not be present • Usually found on the upper trunk, -

Estonian Statistics on Medicines 2013 1/44
Estonian Statistics on Medicines 2013 DDD/1000/ ATC code ATC group / INN (rout of admin.) Quantity sold Unit DDD Unit day A ALIMENTARY TRACT AND METABOLISM 146,8152 A01 STOMATOLOGICAL PREPARATIONS 0,0760 A01A STOMATOLOGICAL PREPARATIONS 0,0760 A01AB Antiinfectives and antiseptics for local oral treatment 0,0760 A01AB09 Miconazole(O) 7139,2 g 0,2 g 0,0760 A01AB12 Hexetidine(O) 1541120 ml A01AB81 Neomycin+Benzocaine(C) 23900 pieces A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+Thymol(dental) 2639 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+Cetylpyridinium chloride(gingival) 179340 g A01AD81 Lidocaine+Cetrimide(O) 23565 g A01AD82 Choline salicylate(O) 824240 pieces A01AD83 Lidocaine+Chamomille extract(O) 317140 g A01AD86 Lidocaine+Eugenol(gingival) 1128 g A02 DRUGS FOR ACID RELATED DISORDERS 35,6598 A02A ANTACIDS 0,9596 Combinations and complexes of aluminium, calcium and A02AD 0,9596 magnesium compounds A02AD81 Aluminium hydroxide+Magnesium hydroxide(O) 591680 pieces 10 pieces 0,1261 A02AD81 Aluminium hydroxide+Magnesium hydroxide(O) 1998558 ml 50 ml 0,0852 A02AD82 Aluminium aminoacetate+Magnesium oxide(O) 463540 pieces 10 pieces 0,0988 A02AD83 Calcium carbonate+Magnesium carbonate(O) 3049560 pieces 10 pieces 0,6497 A02AF Antacids with antiflatulents Aluminium hydroxide+Magnesium A02AF80 1000790 ml hydroxide+Simeticone(O) DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 34,7001 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 3,5364 A02BA02 Ranitidine(O) 494352,3 g 0,3 g 3,5106 A02BA02 Ranitidine(P) -

Persistence of Isoconazole in Vaginal Secretion After Single Application Verweildauer Von Isoconazol Im Vaginalsekret Nach Einmaliger Applikation U
mykosen 27 (2) 97-101 mykosen 1 Eingegangen am 2. November 1983 I 0 Grosse Verlag Berlin 1984 Persistence of Isoconazole in Vaginal Secretion after Single Application Verweildauer von Isoconazol im Vaginalsekret nach einmaliger Applikation U. Tauber, P. Rach and U. Lachnit Forschungslaboratorien der Schering AG BerlidBergkamen Key words: GynoTravogen" vaginal tablets - once-only treatment - drug concentration in vaginal secretion Schliisselworter:Gyno-Travogen" Vaginaltabletten - Einmalapplikation - Wirkstoffspiegel im Vaginalsekret Summary:After intravaginal application of 2 vaginal tablets each containing 300 mg isoconazole nitrate, isoconazole concentration in vaginal secretion above MBC- and MIC-levels of Candida spec. was maintained for at least 72 hours. 7 days after insertion no isoconazole could be detected in the vagina. Obviously the single dose therapy of vaginal mycoses with isoconazole nitrate is as effective and reliable as multi-dose-therapies due to the high long lasting isoconazole levels at the site of infection. Doubts that the high cultural cure rates observed 7 days after application in clinical trials may be caused by still available antimycotic in the vagina are not justified. Zusammenfassung: Nach intravaginaler Applikation von 2 Vaginaltabletten mit jeweils 300 mg Isoconazolnitrat lagen die Isoconazokonzentrationenim Vaginalsekret mindestens 72 Stunden lang iiber den MBK- und MHK-Werten fur Candida-Keime. 7 Tage nach dem Einfien konnte kein Isoconazol mehr in der Vagina nachgewiesen werden. Die mehrere Tage anhaltenden -

Worksheet for Category I: Death After Mpa Injection and (1) Spinal Or
WORKSHEET FOR CATEGORY I: DEATH AFTER MPA INJECTION AND (1) SPINAL OR PARASPINAL FUNGAL INFECTION (including vertebral osteomyelitis, discitis, sacroiliitis, epidural or paraspinal phlegmon, epidural or paraspinal abscess, and/or arachnoiditis); AND/OR (2) FUNGAL MENINGITIS Base Points: 55 Age Adjustment as of Date of Death: 1 point added for each year under 65 (max 20 points) Dependent Children Under 18 as of Date of Death Adjustment: 5 points added for each dependent child under the age of 18 (max 15 points) Spousal Adjustment: 5 points added if married as of Date of Death Adult Children as of Date of Death Adjustment: 3 points added for each surviving adult child (max 9 points) Surgical Debridement or Irrigation Surgery, Laminectomy, Discectomy, or Hemilaminectomy Adjustment: 2 points added for each separate and distinct debridement and/or irrigation surgery without a laminectomy, discectomy, or hemilaminectomy; 4 points for each separate laminectomy, discectomy, or hemilaminectomy whether performed contemporaneously with a debridement and/or irrigation surgery or not. If any laminectomy, discectomy or hemilaminectomy involves multiple vertebral levels, an additional 2 points for that surgery (max 8 points) Anti-Fungal Complication Adjustment: 3 points added for acute renal insufficiency after treatment with amphotericin B; or 5 points added for acute renal insufficiency requiring temporary dialysis after treatment with amphotericin B; or 10 points added for acute renal insufficiency requiring permanent dialysis after treatment with -

Common Study Protocol for Observational Database Studies WP5 – Analytic Database Studies
Arrhythmogenic potential of drugs FP7-HEALTH-241679 http://www.aritmo-project.org/ Common Study Protocol for Observational Database Studies WP5 – Analytic Database Studies V 1.3 Draft Lead beneficiary: EMC Date: 03/01/2010 Nature: Report Dissemination level: D5.2 Report on Common Study Protocol for Observational Database Studies WP5: Conduct of Additional Observational Security: Studies. Author(s): Gianluca Trifiro’ (EMC), Giampiero Version: v1.1– 2/85 Mazzaglia (F-SIMG) Draft TABLE OF CONTENTS DOCUMENT INFOOMATION AND HISTORY ...........................................................................4 DEFINITIONS .................................................... ERRORE. IL SEGNALIBRO NON È DEFINITO. ABBREVIATIONS ......................................................................................................................6 1. BACKGROUND .................................................................................................................7 2. STUDY OBJECTIVES................................ ERRORE. IL SEGNALIBRO NON È DEFINITO. 3. METHODS ..........................................................................................................................8 3.1.STUDY DESIGN ....................................................................................................................8 3.2.DATA SOURCES ..................................................................................................................9 3.2.1. IPCI Database .....................................................................................................9 -

Topical Antifungal Composition Topische Antimykotische Zusammensetzung Composition Antifongique Topique
(19) TZZ __T (11) EP 2 451 468 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 36/53 (2006.01) A61K 36/534 (2006.01) 11.01.2017 Bulletin 2017/02 A61K 36/14 (2006.01) A61K 36/15 (2006.01) A61K 36/23 (2006.01) A61K 36/235 (2006.01) (2006.01) (2006.01) (21) Application number: 10731892.5 A61K 36/54 A61K 36/61 A61K 31/045 (2006.01) A61K 31/05 (2006.01) A61P 31/00 (2006.01) (22) Date of filing: 06.07.2010 (86) International application number: PCT/US2010/041050 (87) International publication number: WO 2011/005749 (13.01.2011 Gazette 2011/02) (54) TOPICAL ANTIFUNGAL COMPOSITION TOPISCHE ANTIMYKOTISCHE ZUSAMMENSETZUNG COMPOSITION ANTIFONGIQUE TOPIQUE (84) Designated Contracting States: (56) References cited: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB WO-A1-2005/087244 WO-A2-96/37210 GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO WO-A2-2004/076680 PL PT RO SE SI SK SM TR • DALLEAU S ET AL: "In vitro activity of essential (30) Priority: 08.07.2009 US 223870 P oils and their major components against Candida albicans yeasts growing planktonically and as (43) Date of publication of application: biofilms" INTERNATIONAL JOURNAL OF 16.05.2012 Bulletin 2012/20 ANTIMICROBIAL AGENTS, vol. 29, no. Suppl. 2, March 2007 (2007-03), page S147, XP22037821 & (73) Proprietor: Onikolabs LLC 17TH EUROPEAN CONGRESS OF CLINICAL Cincinnati, OH 45249-1211 (US) MICROBIOLOGY AND INFECTIOUS DISEASES/25TH INTERNATIONAL CONGRESS; (72) Inventor: BOEGLI, Charles, J.