Cortical Abnormalities in Adults and Adolescents with Major Depression
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
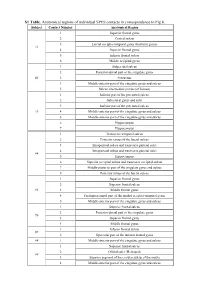
S1 Table. Anatomical Regions of Individual SPES Contacts in Correspondence to Fig 8
S1 Table. Anatomical regions of individual SPES contacts in correspondence to Fig 8. Subject Contact Number Anatomical Region 1 Superior frontal gyrus 2 Central sulcus 3 Lateral occipito-temporal gyrus (fusiform gyrus) #1 4 Superior frontal gyrus 5 Inferior frontal sulcus 6 Middle occipital gyrus 1 Subparietal sulcus 2 Posterior-dorsal part of the cingulate gyrus #2 3 Precuneus 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Sulcus intermedius primus (of Jensen) 1 Inferior part of the precentral sulcus 2 Subcentral gyrus and sulci 3 Inferior part of the precentral sulcus #3 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Middle-anterior part of the cingulate gyrus and sulcus 6 Hippocampus 7 Hippocampus 1 Transverse temporal sulcus 2 Posterior ramus of the lateral sulcus 3 Intraparietal sulcus and transverse parietal sulci 4 Intraparietal sulcus and transverse parietal sulci #4 5 Hippocampus 6 Superior occipital sulcus and transverse occipital sulcus 7 Middle-posterior part of the cingulate gyrus and sulcus 8 Posterior ramus of the lateral sulcus 1 Superior frontal gyrus 2 Superior frontal sulcus #5 3 Middle frontal gyrus 4 Parahippocampal part of the medial occipito-temporal gyrus 5 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Posterior-dorsal part of the cingulate gyrus #6 3 Superior frontal gyrus 4 Middle frontal gyrus 1 Inferior frontal sulcus #7 2 Opercular part of the inferior frontal gyrus #8 1 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Orbital sulci (H-shaped) #9 3 Superior segment of the circular sulcus of the insula 4 Middle-anterior part of the cingulate gyrus and sulcus . -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Insular Volume Reductions in Patients with Major Depressive Disorder
Insular volume reductions in patients with major depressive disorder Item Type Article Authors Mutschler, Isabella; Hänggi, Jürgen; Frei, Manuela; Lieb, Roselind; grosse Holforth, Martin; Seifritz, Erich; Spinelli, Simona Citation Mutschler, I., Hänggi, J., Frei, M., Lieb, R., grosse Holforth, M., Seifritz, E., & Spinelli, S. (2019). Insular volume reductions in patients with major depressive disorder. Neurology, Psychiatry and Brain Research, 33, 39–47. doi:10.1016/j.npbr.2019.06.002 Eprint version Post-print DOI 10.1016/j.npbr.2019.06.002 Publisher Elsevier BV Journal Neurology Psychiatry and Brain Research Rights NOTICE: this is the author’s version of a work that was accepted for publication in Neurology Psychiatry and Brain Research. Changes resulting from the publishing process, such as peer review, editing, corrections, structural formatting, and other quality control mechanisms may not be reflected in this document. Changes may have been made to this work since it was submitted for publication. A definitive version was subsequently published in Neurology Psychiatry and Brain Research, [[Volume], [Issue], (2019-06-22)] DOI: 10.1016/ j.npbr.2019.06.002 . © 2019. This manuscript version is made available under the CC-BY-NC-ND 4.0 license http:// creativecommons.org/licenses/by-nc-nd/4.0/ Download date 23/09/2021 13:26:26 Item License http://creativecommons.org/licenses/by-nc-nd/4.0/ Link to Item http://hdl.handle.net/10754/656271 Neurology, Psychiatry and Brain Research 33 (2019) 39–47 Contents lists available at ScienceDirect Neurology, -

Cortical Abnormalities in Bipolar Disorder: an MRI Analysis of 6503 Individuals from the ENIGMA Bipolar Disorder Working Group
OPEN Molecular Psychiatry (2018) 23, 932–942 www.nature.com/mp ORIGINAL ARTICLE Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group DP Hibar1,2, LT Westlye3,4,5, NT Doan3,4, N Jahanshad1, JW Cheung1, CRK Ching1,6, A Versace7, AC Bilderbeck8, A Uhlmann9,10, B Mwangi11, B Krämer12, B Overs13, CB Hartberg3, C Abé14, D Dima15,16, D Grotegerd17, E Sprooten18, E Bøen19, E Jimenez20, FM Howells9, G Delvecchio21, H Temmingh9, J Starke9, JRC Almeida22, JM Goikolea20, J Houenou23,24, LM Beard25, L Rauer12, L Abramovic26, M Bonnin20, MF Ponteduro16, M Keil27, MM Rive28,NYao29,30, N Yalin31, P Najt32, PG Rosa33,34, R Redlich17, S Trost27, S Hagenaars35, SC Fears36,37, S Alonso-Lana38,39, TGM van Erp40, T Nickson35, TM Chaim-Avancini33,34, TB Meier41,42, T Elvsåshagen3,43, UK Haukvik3,44, WH Lee18, AH Schene45,46, AJ Lloyd47, AH Young31, A Nugent48, AM Dale49,50, A Pfennig51, AM McIntosh35, B Lafer33, BT Baune52, CJ Ekman14, CA Zarate48, CE Bearden53,54, C Henry23,55, C Simhandl56, C McDonald32, C Bourne8,57, DJ Stein9,10, DH Wolf25, DM Cannon32, DC Glahn29,30, DJ Veltman58, E Pomarol-Clotet38,39, E Vieta20, EJ Canales-Rodriguez38,39, FG Nery33,59, FLS Duran33,34, GF Busatto33,34, G Roberts60, GD Pearlson29,30, GM Goodwin8, H Kugel61, HC Whalley35, HG Ruhe8,28,62, JC Soares11, JM Fullerton13,63, JK Rybakowski64, J Savitz42,65, KT Chaim66,67, M Fatjó-Vilas38,39, MG Soeiro-de-Souza33, MP Boks26, MV Zanetti33,34, MCG Otaduy66,67, MS Schaufelberger33,34, M Alda68, M Ingvar14,69, -

Neural Correlates Underlying Change in State Self-Esteem Hiroaki Kawamichi 1,2,3, Sho K
www.nature.com/scientificreports OPEN Neural correlates underlying change in state self-esteem Hiroaki Kawamichi 1,2,3, Sho K. Sugawara2,4,5, Yuki H. Hamano2,5,6, Ryo Kitada 2,7, Eri Nakagawa2, Takanori Kochiyama8 & Norihiro Sadato 2,5 Received: 21 July 2017 State self-esteem, the momentary feeling of self-worth, functions as a sociometer involved in Accepted: 11 January 2018 maintenance of interpersonal relations. How others’ appraisal is subjectively interpreted to change Published: xx xx xxxx state self-esteem is unknown, and the neural underpinnings of this process remain to be elucidated. We hypothesized that changes in state self-esteem are represented by the mentalizing network, which is modulated by interactions with regions involved in the subjective interpretation of others’ appraisal. To test this hypothesis, we conducted task-based and resting-state fMRI. Participants were repeatedly presented with their reputations, and then rated their pleasantness and reported their state self- esteem. To evaluate the individual sensitivity of the change in state self-esteem based on pleasantness (i.e., the subjective interpretation of reputation), we calculated evaluation sensitivity as the rate of change in state self-esteem per unit pleasantness. Evaluation sensitivity varied across participants, and was positively correlated with precuneus activity evoked by reputation rating. Resting-state fMRI revealed that evaluation sensitivity was positively correlated with functional connectivity of the precuneus with areas activated by negative reputation, but negatively correlated with areas activated by positive reputation. Thus, the precuneus, as the part of the mentalizing system, serves as a gateway for translating the subjective interpretation of reputation into state self-esteem. -

Supplementary Tables
Supplementary Tables: ROI Atlas Significant table grey matter Test ROI # Brainetome area beta volume EG pre vs post IT 8 'superior frontal gyrus, part 4 (dorsolateral area 6), right', 0.773 17388 11 'superior frontal gyrus, part 6 (medial area 9), left', 0.793 18630 12 'superior frontal gyrus, part 6 (medial area 9), right', 0.806 24543 17 'middle frontal gyrus, part 2 (inferior frontal junction), left', 0.819 22140 35 'inferior frontal gyrus, part 4 (rostral area 45), left', 1.3 10665 67 'paracentral lobule, part 2 (area 4 lower limb), left', 0.86 13662 EG pre vs post ET 20 'middle frontal gyrus, part 3 (area 46), right', 0.934 28188 21 'middle frontal gyrus, part 4 (ventral area 9/46 ), left' 0.812 27864 31 'inferior frontal gyrus, part 2 (inferior frontal sulcus), left', 0.864 11124 35 'inferior frontal gyrus, part 4 (rostral area 45), left', 1 10665 50 'orbital gyrus, part 5 (area 13), right', -1.7 22626 67 'paracentral lobule, part 2 (area 4 lower limb), left', 1.1 13662 180 'cingulate gyrus, part 3 (pregenual area 32), right', 0.9 10665 261 'Cerebellar lobule VIIb, vermis', -1.5 729 IG pre vs post IT 16 middle frontal gyrus, part 1 (dorsal area 9/46), right', -0.8 27567 24 'middle frontal gyrus, part 5 (ventrolateral area 8), right', -0.8 22437 40 'inferior frontal gyrus, part 6 (ventral area 44), right', -0.9 8262 54 'precentral gyrus, part 1 (area 4 head and face), right', -0.9 14175 64 'precentral gyrus, part 2 (caudal dorsolateral area 6), left', -1.3 18819 81 'middle temporal gyrus, part 1 (caudal area 21), left', -1.4 14472 -

Altered Resting State Connectivity of the Insular Cortex in Individuals with Fibromyalgia
The Journal of Pain, Vol 15, No 8 (August), 2014: pp 815-826 Available online at www.jpain.org and www.sciencedirect.com Original Reports Altered Resting State Connectivity of the Insular Cortex in Individuals With Fibromyalgia Eric Ichesco,* Tobias Schmidt-Wilcke,*,** Rupal Bhavsar,*,y Daniel J. Clauw,* Scott J. Peltier,z Jieun Kim,x Vitaly Napadow,x,{,k Johnson P. Hampson,* Anson E. Kairys,*,yy David A. Williams,* and Richard E. Harris* *Department of Anesthesiology, Chronic Pain and Fatigue Research Center, University of Michigan, Ann Arbor, Michigan. yNeurology Department, University of Pennsylvania, Philadelphia, Pennsylvania. z Functional MRI Laboratory, University of Michigan, Ann Arbor, Michigan. xMGH/MIT/HMS Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, Massachusetts. {Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts. kDepartment of Radiology, Logan College of Chiropractic, Chesterfield, Missouri. **Department of Neurology, Bergmannsheil, Ruhr University Bochum, Bochum, Germany. yyDepartment of Psychology, University of Colorado Denver, Denver, Colorado. Abstract: The insular cortex (IC) and cingulate cortex (CC) are critically involved in pain perception. Previously we demonstrated that fibromyalgia (FM) patients have greater connectivity between the insula and default mode network at rest, and that changes in the degree of this connectivity were associated with changes in the intensity of ongoing clinical pain. In this study we more thoroughly evaluated the degree of resting-state connectivity to multiple regions of the IC in individuals with FM and healthy controls. We also investigated the relationship between connectivity, experimental pain, and current clinical chronic pain. Functional connectivity was assessed using resting-state functional magnetic resonance imaging in 18 FM patients and 18 age- and sex-matched healthy controls using predefined seed regions in the anterior, middle, and posterior IC. -

Seed MNI Coordinates Lobe
MNI Coordinates Seed Lobe (Hemisphere) Region BAa X Y Z FP1 -18 62 0 Frontal Lobe (L) Medial Frontal Gyrus 10 FPz 4 62 0 Frontal Lobe (R) Medial Frontal Gyrus 10 FP2 24 60 0 Frontal Lobe (R) Superior Frontal Gyrus 10 AF7 -38 50 0 Frontal Lobe (L) Middle Frontal Gyrus 10 AF3 -30 50 24 Frontal Lobe (L) Superior Frontal Gyrus 9 AFz 4 58 30 Frontal Lobe (R) Medial Frontal Gyrus 9 AF4 36 48 20 Frontal Lobe (R) Middle Frontal Gyrus 10 AF8 42 46 -4 Frontal Lobe (R) Inferior Frontal Gyrus 10 F7 -48 26 -4 Frontal Lobe (L) Inferior Frontal Gyrus 47 F5 -48 28 18 Frontal Lobe (L) Inferior Frontal Gyrus 45 F3 -38 28 38 Frontal Lobe (L) Precentral Gyrus 9 F1 -20 30 50 Frontal Lobe (L) Superior Frontal Gyrus 8 Fz 2 32 54 Frontal Lobe (L) Superior Frontal Gyrus 8 F2 26 32 48 Frontal Lobe (R) Superior Frontal Gyrus 8 F4 42 30 34 Frontal Lobe (R) Precentral Gyrus 9 F6 50 28 14 Frontal Lobe (R) Middle Frontal Gyrus 46 F8 48 24 -8 Frontal Lobe (R) Inferior Frontal Gyrus 47 FT9 -50 -6 -36 Temporal Lobe (L) Inferior Temporal Gyrus 20 FT7 -54 2 -8 Temporal Lobe (L) Superior Temporal Gyrus 22 FC5 -56 4 22 Frontal Lobe (L) Precentral Gyrus 6 FC3 -44 6 48 Frontal Lobe (L) Middle Frontal Gyrus 6 FC1 -22 6 64 Frontal Lobe (L) Middle Frontal Gyrus 6 FCz 4 6 66 Frontal Lobe (R) Medial Frontal Gyrus 6 FC2 28 8 60 Frontal Lobe (R) Sub-Gyral 6 FC4 48 8 42 Frontal Lobe (R) Middle Frontal Gyrus 6 FC6 58 6 16 Frontal Lobe (R) Inferior Frontal Gyrus 44 FT8 54 2 -12 Temporal Lobe (R) Superior Temporal Gyrus 38 FT10 50 -6 -38 Temporal Lobe (R) Inferior Temporal Gyrus 20 T7/T3 -

Functional Connectivity of the Precuneus in Unmedicated Patients with Depression
Biological Psychiatry: CNNI Archival Report Functional Connectivity of the Precuneus in Unmedicated Patients With Depression Wei Cheng, Edmund T. Rolls, Jiang Qiu, Deyu Yang, Hongtao Ruan, Dongtao Wei, Libo Zhao, Jie Meng, Peng Xie, and Jianfeng Feng ABSTRACT BACKGROUND: The precuneus has connectivity with brain systems implicated in depression. METHODS: We performed the first fully voxel-level resting-state functional connectivity (FC) neuroimaging analysis of depression of the precuneus, with 282 patients with major depressive disorder and 254 control subjects. RESULTS: In 125 unmedicated patients, voxels in the precuneus had significantly increased FC with the lateral orbitofrontal cortex, a region implicated in nonreward that is thereby implicated in depression. FC was also increased in depression between the precuneus and the dorsolateral prefrontal cortex, temporal cortex, and angular and supramarginal areas. In patients receiving medication, the FC between the lateral orbitofrontal cortex and precuneus was decreased back toward that in the control subjects. In the 254 control subjects, parcellation revealed superior anterior, superior posterior, and inferior subdivisions, with the inferior subdivision having high connectivity with the posterior cingulate cortex, parahippocampal gyrus, angular gyrus, and prefrontal cortex. It was the ventral subdivision of the precuneus that had increased connectivity in depression with the lateral orbitofrontal cortex and adjoining inferior frontal gyrus. CONCLUSIONS: The findings support the theory that the system in the lateral orbitofrontal cortex implicated in the response to nonreceipt of expected rewards has increased effects on areas in which the self is represented, such as the precuneus. This may result in low self-esteem in depression. The increased connectivity of the precuneus with the prefrontal cortex short-term memory system may contribute to the rumination about low self-esteem in depression. -

Anatomy and White Matter Connections of the Superior Frontal Gyrus
Anatomy and White Matter Connections of the Superior Frontal Gyrus Robert G. Briggs, BS1; A. Basit Khan, MD5; Arpan R. Chakraborty, BS2; Carol J Abraham, BS2; Christopher D. Anderson, BA2; Patrick J. Karas, MD5; Phillip A. Bonney, MD1; Ali H. Palejwala, MD2; Andrew K. Conner, MD2; Daniel L. O’Donoghue, PhD3; and Michael E. Sughrue, MD4 1Department of Neurosurgery, University of Southern California, Los Angeles, California 2Department of Neurosurgery, University of Oklahoma Health Science Center, Oklahoma City, Oklahoma 3Department of Cell Biology, University of Oklahoma Health Science Center, Oklahoma City, Oklahoma 4Center for Minimally Invasive Neurosurgery, Prince of Wales Private Hospital, Sydney, Australia 5Department of Neurosurgery, Baylor College of Medicine, Houston, Texas Running Title: SFG Subcortical Anatomy Keywords: neurology, neurosurgery, white matter, imaging, connectome Corresponding Author: Michael E. Sughrue, MD Suite 3, Level 7 Prince of Wales Private Hospital Barker Street, Randwick New South Wales, 2031 Australia Tel: 02 9650 4940 Fax: 02 9650 4902 Email: [email protected] This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1002/ca.23523 This article is protected by copyright. All rights reserved. Funding: None. Declaration of Interests: None. Anatomy and White Matter Connections of the Superior Frontal Gyrus ABSTRACT Introduction. The superior frontal gyrus (SFG) is an important region implicated in a variety of tasks including motor movement, working memory, resting-state and cognitive control. A detailed understanding of the subcortical white matter of the SFG could improve post-operative morbidity related to surgery around this gyrus. -

Normal Cortical Anatomy
Normal Cortical Anatomy MGH Massachusetts General Hospital Harvard Medical School NORMAL CORTICAL ANATOMY • Sagittal • Axial • Coronal • The Central Sulcus NP/MGH Sagittal Neuroanatomy NP/MGH Cingulate sulcus Superior frontal gyrus Marginal ramus of Cingulate sulcus Cingulate gyrus Paracentral lobule Superior parietal lobule Parietooccipital sulcus Cuneus Calcarine sulcus Lingual gyrus Subcallosal gyrus Gyrus rectus Fastigium, fourth ventricle NP/MGH Superior frontal gyrus Cingulate sulcus Precentral gyrus Marginal ramus of Cingulate gyrus Central sulcus Cingulate sulcus Superior parietal lobule Precuneus Parietooccipital sulcus Cuneus Calcarine sulcus Frontomarginal gyrus Lingual gyrus Caudothallamic groove Gyrus rectus NP/MGH Precentral sulcus Central sulcus Superior frontal gyrus Marginal ramus of Corona radiata Cingulate sulcus Superior parietal lobule Precuneus Parietooccipital sulcus Calcarine sulcus Inferior occipital gyrus Lingual gyrus NP/MGH Central sulcus Superior parietal lobule Parietooccipital sulcus Frontopolar gyrus Frontomarginal gyrus Superior occipital gyrus Middle occipital gyrus Medial orbital gyrus Lingual gyrus Posterior orbital gyrus Inferior occipital gyrus Inferior temporal gyrus Temporal horn, lateral ventricle NP/MGH Central sulcus Superior Temporal gyrus Middle Temporal gyrus Inferior Temporal gyrus NP/MGH Central sulcus Superior parietal gyrus Inferior frontal gyrus Frontomarginal gyrus Anterior orbital gyrus Superior occipital gyrus Middle occipital Posterior orbital gyrus gyrus Superior Temporal gyrus Inferior -

Supplementary Figure 1 A
100 r = 0.82 100 r = 0.87 100 r = 0.93 p = 0.002 p = 0.001 p < 0.001 90 90 90 80 80 80 VVIQ_1 VVIQ_2 VVIQ_1 70 70 70 60 60 60 55 60 65 70 75 80 85 90 95 100 55 60 65 70 75 80 85 90 95 100 55 60 65 70 75 80 85 90 95 100 VVIQ_2 VVIQ_3 VVIQ_3 100 100 100 r = 0.78 r = 0,85 R = 0.90 90 p = 0.005 90 p = 0.001 90 p < 0.001 80 80 80 70 70 70 VVIQ_S1 VVIQ_S1 60 60 VVIQ_S2 60 50 50 50 40 40 40 40 50 60 70 80 90 100 40 50 60 70 80 90 100 40 50 60 70 80 90 100 VVIQ_S2 VVIQ_S3 VVIQ_S3 Supplementary Figure 1 A. SES (Sensory Component) piercing durchstoßen hot heiss hammering hämmernd stinging stechend glowing glühend throbbing pochend tearing reißend burning brennend knocking klopfend cutting* * schneidend* 0 1 2 3 4 5 B. SES (Affective Component) disabling lähmend unbearable unerträglich terrible furchtbar torturing marternd enervating entnervend heavy schwer nasty scheusslich awful schauderhaft miserable elend murdering mörderisch violent heftig exhaustive erschöpfend cruel grausam excrutiating quälend 0 1 2 3 4 5 Supplementary Figure 2 Supplemental Table 1: Group level (N=10) brain activation during pain Imagination (A.), pain memory (B.) and pain imagination vs. pain memory (C.). A. Pain Imagination Cluster T-contrast Brain area BA x,y,z size t value Activations Right Hemisphere Frontal lobe 56,-2,16 5 4.47 Cerebellum anterior lobe, 2,-46,-6 22 6.48 Vermis 4-5 19 Cerebellum posterior lobe,inferior semi-lunar lobe 12,-72,-50 12 6.11 Activations Left Hemisphere Precentral gyrus (face) -54,-8,38 14 4.65 Postcentral gyrus (tooth) -60,-18,26 30 5.01