Fiber Tracts of the Dorsal Language Stream in the Human Brain
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
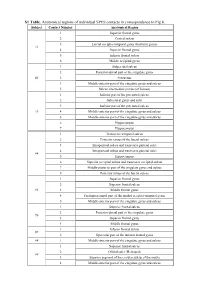
S1 Table. Anatomical Regions of Individual SPES Contacts in Correspondence to Fig 8
S1 Table. Anatomical regions of individual SPES contacts in correspondence to Fig 8. Subject Contact Number Anatomical Region 1 Superior frontal gyrus 2 Central sulcus 3 Lateral occipito-temporal gyrus (fusiform gyrus) #1 4 Superior frontal gyrus 5 Inferior frontal sulcus 6 Middle occipital gyrus 1 Subparietal sulcus 2 Posterior-dorsal part of the cingulate gyrus #2 3 Precuneus 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Sulcus intermedius primus (of Jensen) 1 Inferior part of the precentral sulcus 2 Subcentral gyrus and sulci 3 Inferior part of the precentral sulcus #3 4 Middle-anterior part of the cingulate gyrus and sulcus 5 Middle-anterior part of the cingulate gyrus and sulcus 6 Hippocampus 7 Hippocampus 1 Transverse temporal sulcus 2 Posterior ramus of the lateral sulcus 3 Intraparietal sulcus and transverse parietal sulci 4 Intraparietal sulcus and transverse parietal sulci #4 5 Hippocampus 6 Superior occipital sulcus and transverse occipital sulcus 7 Middle-posterior part of the cingulate gyrus and sulcus 8 Posterior ramus of the lateral sulcus 1 Superior frontal gyrus 2 Superior frontal sulcus #5 3 Middle frontal gyrus 4 Parahippocampal part of the medial occipito-temporal gyrus 5 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Posterior-dorsal part of the cingulate gyrus #6 3 Superior frontal gyrus 4 Middle frontal gyrus 1 Inferior frontal sulcus #7 2 Opercular part of the inferior frontal gyrus #8 1 Middle-anterior part of the cingulate gyrus and sulcus 1 Superior frontal sulcus 2 Orbital sulci (H-shaped) #9 3 Superior segment of the circular sulcus of the insula 4 Middle-anterior part of the cingulate gyrus and sulcus . -

Prefrontal and Posterior Parietal Contributions to the Perceptual Awareness of Touch M
www.nature.com/scientificreports OPEN Prefrontal and posterior parietal contributions to the perceptual awareness of touch M. Rullmann1,2,5, S. Preusser1,5 & B. Pleger1,3,4* Which brain regions contribute to the perceptual awareness of touch remains largely unclear. We collected structural magnetic resonance imaging scans and neurological examination reports of 70 patients with brain injuries or stroke in S1 extending into adjacent parietal, temporal or pre-/frontal regions. We applied voxel-based lesion-symptom mapping to identify brain areas that overlap with an impaired touch perception (i.e., hypoesthesia). As expected, patients with hypoesthesia (n = 43) presented lesions in all Brodmann areas in S1 on postcentral gyrus (BA 1, 2, 3a, 3b). At the anterior border to BA 3b, we additionally identifed motor area BA 4p in association with hypoesthesia, as well as further ventrally the ventral premotor cortex (BA 6, BA 44), assumed to be involved in whole-body perception. At the posterior border to S1, we found hypoesthesia associated efects in attention-related areas such as the inferior parietal lobe and intraparietal sulcus. Downstream to S1, we replicated previously reported lesion-hypoesthesia associations in the parietal operculum and insular cortex (i.e., ventral pathway of somatosensory processing). The present fndings extend this pathway from S1 to the insular cortex by prefrontal and posterior parietal areas involved in multisensory integration and attention processes. Te primary somatosensory cortex (S1) in monkeys can be divided into four Brodmann areas: (BA) 1, 2, 3a, and 3b. Each BA consists of a somatotopically organized map that subserves distinct somatosensory functions1–3. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Insular Volume Reductions in Patients with Major Depressive Disorder
Insular volume reductions in patients with major depressive disorder Item Type Article Authors Mutschler, Isabella; Hänggi, Jürgen; Frei, Manuela; Lieb, Roselind; grosse Holforth, Martin; Seifritz, Erich; Spinelli, Simona Citation Mutschler, I., Hänggi, J., Frei, M., Lieb, R., grosse Holforth, M., Seifritz, E., & Spinelli, S. (2019). Insular volume reductions in patients with major depressive disorder. Neurology, Psychiatry and Brain Research, 33, 39–47. doi:10.1016/j.npbr.2019.06.002 Eprint version Post-print DOI 10.1016/j.npbr.2019.06.002 Publisher Elsevier BV Journal Neurology Psychiatry and Brain Research Rights NOTICE: this is the author’s version of a work that was accepted for publication in Neurology Psychiatry and Brain Research. Changes resulting from the publishing process, such as peer review, editing, corrections, structural formatting, and other quality control mechanisms may not be reflected in this document. Changes may have been made to this work since it was submitted for publication. A definitive version was subsequently published in Neurology Psychiatry and Brain Research, [[Volume], [Issue], (2019-06-22)] DOI: 10.1016/ j.npbr.2019.06.002 . © 2019. This manuscript version is made available under the CC-BY-NC-ND 4.0 license http:// creativecommons.org/licenses/by-nc-nd/4.0/ Download date 23/09/2021 13:26:26 Item License http://creativecommons.org/licenses/by-nc-nd/4.0/ Link to Item http://hdl.handle.net/10754/656271 Neurology, Psychiatry and Brain Research 33 (2019) 39–47 Contents lists available at ScienceDirect Neurology, -

Cortical Abnormalities in Bipolar Disorder: an MRI Analysis of 6503 Individuals from the ENIGMA Bipolar Disorder Working Group
OPEN Molecular Psychiatry (2018) 23, 932–942 www.nature.com/mp ORIGINAL ARTICLE Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group DP Hibar1,2, LT Westlye3,4,5, NT Doan3,4, N Jahanshad1, JW Cheung1, CRK Ching1,6, A Versace7, AC Bilderbeck8, A Uhlmann9,10, B Mwangi11, B Krämer12, B Overs13, CB Hartberg3, C Abé14, D Dima15,16, D Grotegerd17, E Sprooten18, E Bøen19, E Jimenez20, FM Howells9, G Delvecchio21, H Temmingh9, J Starke9, JRC Almeida22, JM Goikolea20, J Houenou23,24, LM Beard25, L Rauer12, L Abramovic26, M Bonnin20, MF Ponteduro16, M Keil27, MM Rive28,NYao29,30, N Yalin31, P Najt32, PG Rosa33,34, R Redlich17, S Trost27, S Hagenaars35, SC Fears36,37, S Alonso-Lana38,39, TGM van Erp40, T Nickson35, TM Chaim-Avancini33,34, TB Meier41,42, T Elvsåshagen3,43, UK Haukvik3,44, WH Lee18, AH Schene45,46, AJ Lloyd47, AH Young31, A Nugent48, AM Dale49,50, A Pfennig51, AM McIntosh35, B Lafer33, BT Baune52, CJ Ekman14, CA Zarate48, CE Bearden53,54, C Henry23,55, C Simhandl56, C McDonald32, C Bourne8,57, DJ Stein9,10, DH Wolf25, DM Cannon32, DC Glahn29,30, DJ Veltman58, E Pomarol-Clotet38,39, E Vieta20, EJ Canales-Rodriguez38,39, FG Nery33,59, FLS Duran33,34, GF Busatto33,34, G Roberts60, GD Pearlson29,30, GM Goodwin8, H Kugel61, HC Whalley35, HG Ruhe8,28,62, JC Soares11, JM Fullerton13,63, JK Rybakowski64, J Savitz42,65, KT Chaim66,67, M Fatjó-Vilas38,39, MG Soeiro-de-Souza33, MP Boks26, MV Zanetti33,34, MCG Otaduy66,67, MS Schaufelberger33,34, M Alda68, M Ingvar14,69, -

Neural Correlates Underlying Change in State Self-Esteem Hiroaki Kawamichi 1,2,3, Sho K
www.nature.com/scientificreports OPEN Neural correlates underlying change in state self-esteem Hiroaki Kawamichi 1,2,3, Sho K. Sugawara2,4,5, Yuki H. Hamano2,5,6, Ryo Kitada 2,7, Eri Nakagawa2, Takanori Kochiyama8 & Norihiro Sadato 2,5 Received: 21 July 2017 State self-esteem, the momentary feeling of self-worth, functions as a sociometer involved in Accepted: 11 January 2018 maintenance of interpersonal relations. How others’ appraisal is subjectively interpreted to change Published: xx xx xxxx state self-esteem is unknown, and the neural underpinnings of this process remain to be elucidated. We hypothesized that changes in state self-esteem are represented by the mentalizing network, which is modulated by interactions with regions involved in the subjective interpretation of others’ appraisal. To test this hypothesis, we conducted task-based and resting-state fMRI. Participants were repeatedly presented with their reputations, and then rated their pleasantness and reported their state self- esteem. To evaluate the individual sensitivity of the change in state self-esteem based on pleasantness (i.e., the subjective interpretation of reputation), we calculated evaluation sensitivity as the rate of change in state self-esteem per unit pleasantness. Evaluation sensitivity varied across participants, and was positively correlated with precuneus activity evoked by reputation rating. Resting-state fMRI revealed that evaluation sensitivity was positively correlated with functional connectivity of the precuneus with areas activated by negative reputation, but negatively correlated with areas activated by positive reputation. Thus, the precuneus, as the part of the mentalizing system, serves as a gateway for translating the subjective interpretation of reputation into state self-esteem. -

Down but Not out in Posterior Cingulate Cortex: Deactivation Yet Functional Coupling with Prefrontal Cortex During Demanding Semantic Cognition
NeuroImage 141 (2016) 366–377 Contents lists available at ScienceDirect NeuroImage journal homepage: www.elsevier.com/locate/ynimg Down but not out in posterior cingulate cortex: Deactivation yet functional coupling with prefrontal cortex during demanding semantic cognition Katya Krieger-Redwood ⁎, Elizabeth Jefferies, Theodoros Karapanagiotidis, Robert Seymour, Adonany Nunes, Jit Wei Aaron Ang, Vierra Majernikova, Giovanna Mollo, Jonathan Smallwood Department of Psychology/York Neuroimaging Centre, University of York, Heslington, York, United Kingdom article info abstract Article history: The posterior cingulate cortex (pCC) often deactivates during complex tasks, and at rest is often only weakly cor- Received 30 March 2016 related with regions that play a general role in the control of cognition. These observations led to the hypothesis Accepted 29 July 2016 that pCC contributes to automatic aspects of memory retrieval and cognition. Recent work, however, has sug- Available online 30 July 2016 gested that the pCC may support both automatic and controlled forms of memory processing and may do so by changing its communication with regions that are important in the control of cognition across multiple do- Keywords: mains. The current study examined these alternative views by characterising the functional coupling of the Posterior cingulate cortex Dorsolateral prefrontal cortex pCC in easy semantic decisions (based on strong global associations) and in harder semantic tasks (matching Semantic control words on the basis of specific non-dominant features). Increasingly difficult semantic decisions led to the expect- Executive ed pattern of deactivation in the pCC; however, psychophysiological interaction analysis revealed that, under Rest these conditions, the pCC exhibited greater connectivity with dorsolateral prefrontal cortex (PFC), relative to Connectivity both easier semantic decisions and to a period of rest. -

Potential Scalp Stimulation Targets for Mental Disorders
Cao et al. J Transl Med (2021) 19:343 https://doi.org/10.1186/s12967-021-02993-1 Journal of Translational Medicine RESEARCH Open Access Potential scalp stimulation targets for mental disorders: evidence from neuroimaging studies Jin Cao, Thalia Celeste Chai‑Zhang, Yiting Huang, Maya Nicole Eshel and Jian Kong* Abstract Mental disorders widely contribute to the modern global disease burden, creating a signifcant need for improvement of treatments. Scalp stimulation methods (such as scalp acupuncture and transcranial electrical stimulation) have shown promising results in relieving psychiatric symptoms. However, neuroimaging fndings haven’t been well‑ integrated into scalp stimulation treatments. Identifying surface brain regions associated with mental disorders would expand target selection and the potential for these interventions as treatments for mental disorders. In this study, we performed large‑scale meta‑analyses separately on eight common mental disorders: attention defcit hyperactivity disorder, anxiety disorder, autism spectrum disorder, bipolar disorder, compulsive disorder, major depression, post‑ traumatic stress disorder and schizophrenia; utilizing modern neuroimaging literature to summarize disorder‑asso‑ ciated surface brain regions, and proposed neuroimaging‑based target protocols. We found that the medial frontal gyrus, the supplementary motor area, and the dorsal lateral prefrontal cortex are commonly involved in the patho‑ physiology of mental disorders. The target protocols we proposed may provide new brain targets for scalp stimulation in the treatment of mental disorders, and facilitate its clinical application. Keywords: Neuroimaging, Meta‑analysis, Scalp stimulation, Scalp acupuncture, Transcranial electrical stimulation, Mental disorder Introduction For instance, scalp acupuncture, a modern school of Mental disorders are a major component of the modern acupuncture developed on the basis of anatomical and global disease burden. -

Keeping Order in the Brain: the Supramarginal Gyrus and Serial Order in Short-Term Memory
cortex 119 (2019) 89e99 Available online at www.sciencedirect.com ScienceDirect Journal homepage: www.elsevier.com/locate/cortex Research Report Keeping order in the brain: The supramarginal gyrus and serial order in short-term memory Giacomo Guidalia,b, Alberto Pisonia, Nadia Bologninia,c and * Costanza Papagnoa,d, a Department of Psychology & Milan Center for Neuroscience - NeuroMI, University of Milano-Bicocca, Milan, Italy b Ph.D. program in Neuroscience, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy c Laboratory of Neuropsychology, IRCCS Istituto Auxologico Italiano, Milan, Italy d CIMeC & CeRiN, University of Trento, Rovereto, Italy article info abstract Article history: A wide range of human activities are performed sequentially in few seconds. We need to Received 13 December 2018 maintain a correct temporal order of words in language, movements in actions, directions Reviewed 5 February 2019 in navigation, etc. Therefore, it is plausible, in a more economical perspective, that our Revised 22 February 2019 brain is equipped with a dedicated mechanism for storing a temporal sequence for a short Accepted 10 April 2019 time. To investigate it, we run four TMS experiments, in which participants performed Action editor Eric Wassermann different short-term memory tasks, i.e., three (verbal, spatial, motor) requiring mainte- Published online 20 April 2019 nance of an ordered sequence and one (visual) of a static pattern. We demonstrated, for the first time, that the left supramarginal gyrus is one of the key nodes of the STM network Keywords: involved in retaining an abstract representation of serial order information, independently Short-term memory from the content information, namely the nature of the item to be remembered, which Serial order instead is stored separately. -

1. Lateral View of Lobes in Left Hemisphere TOPOGRAPHY
TOPOGRAPHY T1 Division of Cerebral Cortex into Lobes 1. Lateral View of Lobes in Left Hemisphere 2. Medial View of Lobes in Right Hemisphere PARIETAL PARIETAL LIMBIC FRONTAL FRONTAL INSULAR: buried OCCIPITAL OCCIPITAL in lateral fissure TEMPORAL TEMPORAL 3. Dorsal View of Lobes 4. Ventral View of Lobes PARIETAL TEMPORAL LIMBIC FRONTAL OCCIPITAL FRONTAL OCCIPITAL Comment: The cerebral lobes are arbitrary divisions of the cerebrum, taking their names, for the most part, from overlying bones. They are not functional subdivisions of the brain, but serve as a reference for locating specific functions within them. The anterior (rostral) end of the frontal lobe is referred to as the frontal pole. Similarly, the anterior end of the temporal lobe is the temporal pole, and the posterior end of the occipital lobe the occipital pole. TOPOGRAPHY T2 central sulcus central sulcus parietal frontal occipital lateral temporal lateral sulcus sulcus SUMMARY CARTOON: LOBES SUMMARY CARTOON: GYRI Lateral View of Left Hemisphere central sulcus postcentral superior parietal superior precentral gyrus gyrus lobule frontal intraparietal sulcus gyrus inferior parietal lobule: supramarginal and angular gyri middle frontal parieto-occipital sulcus gyrus incision for close-up below OP T preoccipital O notch inferior frontal cerebellum gyrus: O-orbital lateral T-triangular sulcus superior, middle and inferior temporal gyri OP-opercular Lateral View of Insula central sulcus cut surface corresponding to incision in above figure insula superior temporal gyrus Comment: Insula (insular gyri) exposed by removal of overlying opercula (“lids” of frontal and parietal cortex). TOPOGRAPHY T3 Language sites and arcuate fasciculus. MRI reconstruction from a volunteer. central sulcus supramarginal site (posterior Wernicke’s) Language sites (squares) approximated from electrical stimulation sites in patients undergoing operations for epilepsy or tumor removal (Ojeman and Berger). -

Supplementary Tables
Supplementary Tables: ROI Atlas Significant table grey matter Test ROI # Brainetome area beta volume EG pre vs post IT 8 'superior frontal gyrus, part 4 (dorsolateral area 6), right', 0.773 17388 11 'superior frontal gyrus, part 6 (medial area 9), left', 0.793 18630 12 'superior frontal gyrus, part 6 (medial area 9), right', 0.806 24543 17 'middle frontal gyrus, part 2 (inferior frontal junction), left', 0.819 22140 35 'inferior frontal gyrus, part 4 (rostral area 45), left', 1.3 10665 67 'paracentral lobule, part 2 (area 4 lower limb), left', 0.86 13662 EG pre vs post ET 20 'middle frontal gyrus, part 3 (area 46), right', 0.934 28188 21 'middle frontal gyrus, part 4 (ventral area 9/46 ), left' 0.812 27864 31 'inferior frontal gyrus, part 2 (inferior frontal sulcus), left', 0.864 11124 35 'inferior frontal gyrus, part 4 (rostral area 45), left', 1 10665 50 'orbital gyrus, part 5 (area 13), right', -1.7 22626 67 'paracentral lobule, part 2 (area 4 lower limb), left', 1.1 13662 180 'cingulate gyrus, part 3 (pregenual area 32), right', 0.9 10665 261 'Cerebellar lobule VIIb, vermis', -1.5 729 IG pre vs post IT 16 middle frontal gyrus, part 1 (dorsal area 9/46), right', -0.8 27567 24 'middle frontal gyrus, part 5 (ventrolateral area 8), right', -0.8 22437 40 'inferior frontal gyrus, part 6 (ventral area 44), right', -0.9 8262 54 'precentral gyrus, part 1 (area 4 head and face), right', -0.9 14175 64 'precentral gyrus, part 2 (caudal dorsolateral area 6), left', -1.3 18819 81 'middle temporal gyrus, part 1 (caudal area 21), left', -1.4 14472 -

Quasi-Periodic Patterns Contribute to Functional Connectivity in the Brain
bioRxiv preprint doi: https://doi.org/10.1101/323162; this version posted May 16, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Quasi-periodic patterns contribute to functional connectivity in the brain Anzar Abbasa, Michaël Belloyb, Amrit Kashyapc, Jacob Billingsa, Maysam Nezafatic, Shella Keilholza,c a Neuroscience, Emory University, 1760 Haygood Dr NE Suite W-200, Atlanta, GA 30322, United States b Bio-Imaging Lab, University of Antwerp, Universiteitsplein 1, 2610 Wilrijk, Antwerp, Belgium c Biomedical Engineering, Emory University and Georgia Institute of Technology, 1760 Haygood Dr NE Suite W-200, Atlanta, GA 30322, United States Corresponding Author Shella Keilholz [email protected] 1760 Haygood Dr NE W-230 Atlanta, GA 30322 Highlights • Quasi-periodic patterns (QPPs) of low-frequency activity contribute to functional connectivity • The spatiotemporal pattern of QPPs differs between resting-state and task-performing individuals • QPPs account for significant functional connectivity in the DMN and TPN during rest and task performance • Changes in functional connectivity in these networks may actually reflect differences in the QPPs Declarations of Interest None !1 bioRxiv preprint doi: https://doi.org/10.1101/323162; this version posted May 16, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license.