M.Sc. (Chemistry) I-IV Semester Course and Examination Structure M.Sc. Previous – First Semester Maximum Marks CHE 101 Paper
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Planar Cyclopenten‐4‐Yl Cations: Highly Delocalized Π Aromatics
Angewandte Research Articles Chemie How to cite: Angew.Chem. Int. Ed. 2020, 59,18809–18815 Carbocations International Edition: doi.org/10.1002/anie.202009644 German Edition: doi.org/10.1002/ange.202009644 Planar Cyclopenten-4-yl Cations:Highly Delocalized p Aromatics Stabilized by Hyperconjugation Samuel Nees,Thomas Kupfer,Alexander Hofmann, and Holger Braunschweig* 1 B Abstract: Theoretical studies predicted the planar cyclopenten- being energetically favored by 18.8 kcalmolÀ over 1 (MP3/ 4-yl cation to be aclassical carbocation, and the highest-energy 6-31G**).[11–13] Thebishomoaromatic structure 1B itself is + 1 isomer of C5H7 .Hence,its existence has not been verified about 6–14 kcalmolÀ lower in energy (depending on the level experimentally so far.Wewere now able to isolate two stable of theory) than the classical planar structure 1C,making the derivatives of the cyclopenten-4-yl cation by reaction of bulky cyclopenten-4-yl cation (1C)the least favorable isomer.Early R alanes Cp AlBr2 with AlBr3.Elucidation of their (electronic) solvolysis studies are consistent with these findings,with structures by X-raydiffraction and quantum chemistry studies allylic 1A being the only observable isomer, notwithstanding revealed planar geometries and strong hyperconjugation the nature of the studied cyclopenteneprecursor.[14–18] Thus, interactions primarily from the C Al s bonds to the empty p attempts to generate isomer 1C,orits homoaromatic analog À orbital of the cationic sp2 carbon center.Aclose inspection of 1B,bysolvolysis of 4-Br/OTs-cyclopentene -

An Analysis of Electrophilic Aromatic Substitution: a “Complex Approach” PCCP
Volume 23 Number 9 7 March 2021 Pages 5033–5682 PCCP Physical Chemistry Chemical Physics rsc.li/pccp ISSN 1463-9076 PERSPECTIVE Janez Cerkovnik et al . An analysis of electrophilic aromatic substitution: a “complex approach” PCCP View Article Online PERSPECTIVE View Journal | View Issue An analysis of electrophilic aromatic substitution: a ‘‘complex approach’’† Cite this: Phys. Chem. Chem. Phys., a a b 2021, 23, 5051 Nikola Stamenkovic´, Natasˇa Poklar Ulrih and Janez Cerkovnik * Electrophilic aromatic substitution (EAS) is one of the most widely researched transforms in synthetic organic chemistry. Numerous studies have been carried out to provide an understanding of the nature of its reactivity pattern. There is now a need for a concise and general, but detailed and up-to-date, overview. The basic principles behind EAS are essential to our understanding of what the mechanisms underlying EAS are. To date, textbook overviews of EAS have provided little information about the Received 5th October 2020, mechanistic pathways and chemical species involved. In this review, the aim is to gather and present the Accepted 21st December 2020 up-to-date information relating to reactivity in EAS, with the implication that some of the key concepts DOI: 10.1039/d0cp05245k will be discussed in a scientifically concise manner. In addition, the information presented herein suggests certain new possibilities to advance EAS theory, with particular emphasis on the role of modern Creative Commons Attribution-NonCommercial 3.0 Unported Licence. rsc.li/pccp -

FAROOK COLLEGE (Autonomous)
FAROOK COLLEGE (Autonomous) M.Sc. DEGREE PROGRAMME IN CHEMISTRY CHOICE BASED CREDIT AND SEMESTER SYSTEM-PG (FCCBCSSPG-2019) SCHEME AND SYLLABI 2019 ADMISSION ONWARDS 1 CERTIFICATE I hereby certify that the documents attached are the bona fide copies of the syllabus of M.Sc. Chemistry Programme to be effective from the academic year 2019-20 onwards. Date: Place: P R I N C I P A L 2 FAROOK COLLEGE (AUTONOMOUS) MSc. CHEMISTRY (CSS PATTERN) Regulations and Syllabus with effect from 2019 admission Pattern of the Programme a. The name of the programme shall be M.Sc. Chemistry under CSS pattern. b. The programme shall be offered in four semesters within a period of two academic years. c. Eligibility for admission will be as per the rules laid down by the College from time to time. d. Details of the courses offered for the programme are given in Table 1. The programme shall be conducted in accordance with the programme pattern, scheme of examination and syllabus prescribed. Of the 25 hours per week, 13 hours shall be allotted for theory and 12 hours for practical; 1 theory hour per week during even semesters shall be allotted for seminar. Theory Courses In the first three semesters, there will be four theory courses; and in the fourth semester, three theory courses. All the theory courses in the first and second semesters are core courses. In the third semester there will be three core theory courses and one elective theory course. College can choose any one of the elective courses given in Table 1. -

Organometallic Compounds
Chapter 11 Organometallic compounds Organometallics Reactions using organometallics Organometallic compounds Ch 11 #2 = comp’s containing a carbon-metal bond δ− δ+ C in organometallic comp’ds are nucleophilic. C in organic comp’d (like ROH, RNH , and RX) 2 δ+ δ are electrophilic. − due to ∆EN Ch 11 #3 in substitution reactions a carbon Nu: R-Li and R-MgX Ch 11 #4 (used to be) the two most common organometallics organolithium comp’ds BuLi = an alkyl lithium organomagnesium comp’ds = Grignard reagents 1912 Nobel Prize R, Ar, vinyl all possible; Br (as X) popular Ether (solvent) coordinates Mg, stabilizing it. Reactions of R-Li and R-MgX Ch 11 #5 reacts like a carbanion C:– ~ a C Nu: reactions as C Nu: (like SN(2)) nucleophilic addition to carbonyls ~ more often Chapt 16 Ch 11 #6 R-Li and R-MgX are very strong B: how strong? pKa? − − react even with very weak acid stronger than OH? NH2? useful for preparing deuterated HC Storage and reaction must be acid- and moisture-free. Transmetal(l)ation Ch 11 #7 R-Li is more reactive than R-MgX is. C–Li more polar than C–Mg C of R-Li more nu-philic [better Nu:] transmetalation [metal exchange] to less reactive [more stable] organometallic Coupling using Gilman reagent Ch 11 #8 coupling reaction (in organic chemistry) two hydrocarbon fragments are coupled (to form C–C) with the aid of a (transition) metal catalyst Gilman reagent coupling of R of R-X and R’ of Gilman reagent RX + R’2CuLi R–R’ mechanism? substitution of X with R’? not clear Ch 11 #9 R can be alkyl, aryl, or alkenyl [vinyl] which is not possible by R-Li or R-MgX Is R of Gilman why? they are SN(2). -

Structures and Reaction Mechanisms of Organocuprate Clusters in Organic Chemistry
REVIEWS Wherefore Art Thou Copper? Structures and Reaction Mechanisms of Organocuprate Clusters in Organic Chemistry Eiichi Nakamura* and Seiji Mori Organocopper reagents provide the principles. This review will summarize example of molecular recognition and most general synthetic tools in organic first the general structural features of supramolecular chemistry, which chemistry for nucleophilic delivery of organocopper compounds and the pre- chemists have long exploited without hard carbanions to electrophilic car- vious mechanistic arguments, and then knowing it. Reasoning about the bon centers. A number of structural describe the most recent mechanistic uniqueness of the copper atom among and mechanistic studies have been pictures obtained through high-level neighboring metal elements in the reported and have led to a wide variety quantum mechanical calculations for periodic table will be presented. of mechanistic proposals, some of three typical organocuprate reactions, which might even be contradictory to carbocupration, conjugate addition, Keywords: catalysis ´ conjugate addi- others. With the recent advent of and SN2 alkylation. The unified view tions ´ copper ´ density functional physical and theoretical methodolo- on the nucleophilic reactivities of met- calculations ´ supramolecular chemis- gies, the accumulated knowledge on al organocuprate clusters thus ob- try organocopper chemistry is being put tained has indicated that organocup- together into a few major mechanistic rate chemistry represents an intricate 1. Introduction 1 R Cu X The desire to learn about the nature of elements has been R or R1 R and will remain a main concern of chemists. In this review, we R Cu will consider what properties of copper make organocopper R1 chemistry so useful in organic chemistry. -

Organometrallic Chemistry
CHE 425: ORGANOMETALLIC CHEMISTRY SOURCE: OPEN ACCESS FROM INTERNET; Striver and Atkins Inorganic Chemistry Lecturer: Prof. O. G. Adeyemi ORGANOMETALLIC CHEMISTRY Definitions: Organometallic compounds are compounds that possess one or more metal-carbon bond. The bond must be “ionic or covalent, localized or delocalized between one or more carbon atoms of an organic group or molecule and a transition, lanthanide, actinide, or main group metal atom.” Organometallic chemistry is often described as a bridge between organic and inorganic chemistry. Organometallic compounds are very important in the chemical industry, as a number of them are used as industrial catalysts and as a route to synthesizing drugs that would not have been possible using purely organic synthetic routes. Coordinative unsaturation is a term used to describe a complex that has one or more open coordination sites where another ligand can be accommodated. Coordinative unsaturation is a very important concept in organotrasition metal chemistry. Hapticity of a ligand is the number of atoms that are directly bonded to the metal centre. Hapticity is denoted with a Greek letter η (eta) and the number of bonds a ligand has with a metal centre is indicated as a superscript, thus η1, η2, η3, ηn for hapticity 1, 2, 3, and n respectively. Bridging ligands are normally preceded by μ, with a subscript to indicate the number of metal centres it bridges, e.g. μ2–CO for a CO that bridges two metal centres. Ambidentate ligands are polydentate ligands that can coordinate to the metal centre through one or more atoms. – – – For example CN can coordinate via C or N; SCN via S or N; NO2 via N or N. -

Bond Distances and Bond Orders in Binuclear Metal Complexes of the First Row Transition Metals Titanium Through Zinc
Metal-Metal (MM) Bond Distances and Bond Orders in Binuclear Metal Complexes of the First Row Transition Metals Titanium Through Zinc Richard H. Duncan Lyngdoh*,a, Henry F. Schaefer III*,b and R. Bruce King*,b a Department of Chemistry, North-Eastern Hill University, Shillong 793022, India B Centre for Computational Quantum Chemistry, University of Georgia, Athens GA 30602 ABSTRACT: This survey of metal-metal (MM) bond distances in binuclear complexes of the first row 3d-block elements reviews experimental and computational research on a wide range of such systems. The metals surveyed are titanium, vanadium, chromium, manganese, iron, cobalt, nickel, copper, and zinc, representing the only comprehensive presentation of such results to date. Factors impacting MM bond lengths that are discussed here include (a) n+ the formal MM bond order, (b) size of the metal ion present in the bimetallic core (M2) , (c) the metal oxidation state, (d) effects of ligand basicity, coordination mode and number, and (e) steric effects of bulky ligands. Correlations between experimental and computational findings are examined wherever possible, often yielding good agreement for MM bond lengths. The formal bond order provides a key basis for assessing experimental and computationally derived MM bond lengths. The effects of change in the metal upon MM bond length ranges in binuclear complexes suggest trends for single, double, triple, and quadruple MM bonds which are related to the available information on metal atomic radii. It emerges that while specific factors for a limited range of complexes are found to have their expected impact in many cases, the assessment of the net effect of these factors is challenging. -

Metal-Ligand Bonding and Inorganic Reaction Mechanisms Year 2
Metal-Ligand Bonding and Inorganic Reaction Mechanisms Year 2 RED Metal-ligand and metal-metal bonding of the transition metal elements Synopsis Lecture 1: Trends of the transition metal series. Ionic vs Covalent bonding. Nomenclature. Electron counting. Lecture 2: Thermodynamics of complex formation. Why complexes form. Recap of molecular orbital theory. 18-electron rule. Lecture 3: Ligand classes. -donor complexes. Octahedral ML6 molecular orbital energy diagram. Lecture 3: - acceptor ligands and synergic bonding. Binding of CO, CN , N2, O2 and NO. Lecture 4: Alkenes, M(H2) vs M(H)2, Mn(O2) complexes, PR3. Lecture 5: 2- - 2- 3- donor ligands, metal-ligand multiple bonds, O , R2N , RN , N . Lecture 6: ML6 molecular orbital energy diagrams incorporating acceptor and donor ligands. Electron counting revisited and link to spectrochemical series. Lecture 7: Kinetics of complex formation. Substitution mechanisms of inorganic complexes. Isomerisation. Lecture 8: Ligand effects on substitution rates (trans-effect, trans-influence). Metal and geometry effects on substitution rates. Lecture 9: Outer sphere electron transfer. Lecture 10: Inner Sphere electron transfer. Bridging ligands. 2 Learning Objectives: by the end of the course you should be able to i) Use common nomenclature in transition metal chemistry. ii) Count valence electrons and determine metal oxidation state in transition metal complexes. iii) Understand the physical basis of the 18-electron rule. iv) Appreciate the synergic nature of bonding in metal carbonyl complexes. v) Understand the relationship between CO, the 'classic' -acceptor and related ligands such as NO, CN, N2, and alkenes. 2 vi) Describe the nature of the interaction between -bound diatomic molecules (H2, O2) and their relationship to -acceptor ligands. -

Organocuprate Aggregation and Reactivity: Decoding the 'Black Box'
Dissertation zur Erlangung des Doktorgrades der Fakultät für Chemie und Pharmazie der Ludwig-Maximilians-Universität München Organocuprate Aggregation and Reactivity: Decoding the ‘Black Box’ Aliaksei Putau aus Vitebsk, Weißrussland 2012 Erklärung Diese Dissertation wurde im Sinne von § 7 der Promotionsordnung vom 28. November 2011 von Herrn Prof. Dr. Konrad Koszinowski betreut, und von Herrn Prof. Dr. Mayr von der Fakultät für Chemie und Pharmazie vertreten. Eidesstattliche Versicherung Diese Dissertation wurde selbständig, ohne unerlaubte Hilfe erarbeitet. München, 11.06.2012 _______________________ Aliaksei Putau Dissertation eingereicht am: 12.06.2012 1. Gutachter Prof. Dr. Herbert Mayr 2. Gutachter Prof. Dr. Konrad Koszinowski Mündliche Prüfung am: 20.07.2012 Acknowledgements It is a pleasure to thank the many people who made this project possible. My gratitude to my PhD supervisor, Prof. Dr. Konrad Koszinowski, is difficult to overstate. His sound advice, good company, and enthusiasm for trying new things ensured the tropical sunny climate in our group that is so necessary for fruitful (and, above all, enjoyable) research. Besides, his involvement in the group life outside the lab made all of us group members feel like team members, not just a loose bunch of colleagues. I am also deeply indebted to Prof. Dr. Herbert Mayr, not only for his continuous generous support and help, but also for running such a cool, all-star research group, which provided a great many social occasions. I thank SFB 749 for the financial support of this project, specifically Mrs. Birgit Carell, for her support and understanding of my worries and troubles of all kinds. Furthermore, my sincere gratitude goes to Prof. -
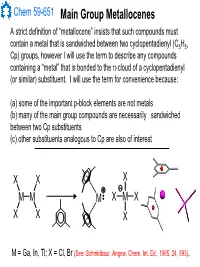
Main Group Metallocenes
Chem 59-651 Main Group Metallocenes A strict definition of “metallocene” insists that such compounds must contain a metal that is sandwiched between two cyclopentadienyl (C5H5, Cp) groups, however I will use the term to describe any compounds containing a “metal” that is bonded to the π-cloud of a cyclopentadienyl (or similar) substituent. I will use the term for convenience because: (a) some of the important p-block elements are not metals (b) many of the main group compounds are necessarily sandwiched between two Cp substituents (c) other substituents analogous to Cp are also of interest XX X MM M XMX X X X M = Ga, In, Tl; X = Cl, Br (See: Schmidbaur, Angew. Chem. Int. Ed., 1985, 24, 893). Chem 59-651 Cyclopentadienyl Ligand Basics The structural features and bonding interactions between cyclopentadienyl groups and metal atoms shows considerable variation and is described using a few different conventions (See Jutzi and Burford, Chem. Rev., 1999, 99, 969 and references therein). In most main group metallocenes, even those that are σ-bonded in the solid state, the rings rotate rapidly through a series of 1,2-shifts. A Cp group can be considered to be p-bonded to an element if the Hapticity: element sits inside the cylinder defined by the 5 carbon atoms of M MM the Cp ligand. The center of mass of the 5 C atoms is known as the centroid. η1-Cp η2-Cp η3-Cp M M M A σ-bonded Cp can be identified η4-Cp η5-Cp ≡η5-Cp by the angle at the ipso carbon M and bond lengths should indicate single bonds to the ipso carbon and a localized diene structure for σ the α and β carbon fragment. -

Application of the Covalent Bond Classification Method for The
Article pubs.acs.org/jchemeduc Application of the Covalent Bond Classification Method for the Teaching of Inorganic Chemistry Malcolm L. H. Green† and Gerard Parkin*,‡ † Inorganic Chemistry Laboratory, University of Oxford, South Parks Road, Oxford OX1 3QR, United Kingdom ‡ Department of Chemistry, Columbia University, New York, New York 10027, United States *S Supporting Information ABSTRACT: The Covalent Bond Classification (CBC) method provides a means to classify covalent molecules according to the number and types of bonds that surround an atom of interest. This approach is based on an elementary molecular orbital analysis of the bonding involving the central atom (M), with the various interactions being classified according to the number of electrons that each neutral ligand contributes to the bonding orbital. Thus, with respect to the atom of interest (M), the ligand can contribute either two (L), one (X), or zero (Z) electrons to a bonding orbital. A normal covalent bond is represented as M−X, whereas dative covalent bonds are represented as either M←LorM→Z, according to whether the ligand is the donor (L) fi or acceptor (Z). A molecule is classi ed as [MLlXxZz] according to the number of L, X, and Z ligand functions that surround M. Not only does fi fi the [MLlXxZz] designation provide a formal classi cation of a molecule, but it also indicates the electron con guration, the valence, and the number of nonbonding electrons on M. As such, the classification allows a student to understand relationships between molecules, thereby increasing their ability to conceptualize and learn the chemistry of the elements. -

Select Reactions of Organoboranes and Organostannanes
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 8-2012 Select Reactions of Organoboranes and Organostannanes David W. Blevins [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Organic Chemistry Commons Recommended Citation Blevins, David W., "Select Reactions of Organoboranes and Organostannanes. " PhD diss., University of Tennessee, 2012. https://trace.tennessee.edu/utk_graddiss/1412 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by David W. Blevins entitled "Select Reactions of Organoboranes and Organostannanes." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Doctor of Philosophy, with a major in Chemistry. George W. Kabalka, Major Professor We have read this dissertation and recommend its acceptance: Shane Foister, Craig Barnes, Kimberly Gwinn Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) Select Reactions of Organoboranes and Organostannanes A Dissertation Presented for the Doctor of Philosophy Degree The University of Tennessee, Knoxville David W. Blevins August 2012 DEDICATION I dedicate this work to Lisa, Preston, and Clarissa Blevins. ii ACKNOWLEDGEMENTS I would like to thank Dr.