Arts, Commerce & Science College, Bodwad
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2-Adamantylacetic Acid* B
CROATICA CHEM1CA ACTA CcACAA 48 (2) I69-i78 (1976) YU ISSN 0011-1643 CCA-927 547.466:542.91 Originai Scientific Paper Synthesis of a-Amino-1-Adamantylacetic and a-Amino -2-Adamantylacetic Acid* B . Gaspert, S. Hromadko, and B. Vranesic Research Department »Piiva«, Pharmaceuticai and Chemicai Works, Zagreb, Croatia, Yugosiavia Rece ived September 29 , 1975 a-Amino-2-adamantylacetic acid (VII) was obtained when 2-adamantylcyanoacetylhydrazide (IX) was subjected to Curtius rearrangement or 2-(2-adaman;tyl)-malonamic acid (XII) to Hof mann degradation. The same amino acid was obtained when ri. -bromo-2-adamantylacetic acid (VI) was •treated with ammonia. Partial hydrolysis of diethyl adamantyl-(1)-malonate (XIV) yie1ded ethyl adamantyl-(1)-malcmate (XV) which, after treatment with thionyl chlodde and then ammonia, gave 2-(1-adamantyl) -malonamic acid ethyl ester (XVII). Hofmann degradation of 2-(1 -adamantyl)-malonamic acid ethyl ester gave a-amino-1- -adama:ntylacetic acid (XVIII). Discovery of interesting biological properties of 1-aminoadamantane hy drochloride' ·stimulated the synthesis of a large number of structurally related compounds. The synthesis of amino acids, having an adamantyl group as a part of the 2 molecule, has been reported for 3-aminoadamantane-1-carboxyHc acid , a -amino-1-adamantylacetic acid3 and recently for 2-'aminoadamantane-2-car boxylic acid4. It seemed appmpriate to us to synthetise a-amLno-2-adamantyl acetic acid5 and to elaborate a moire convenient synthesis of a-amino-1-ada ma:ntylacetic acid6, which -

Synthesis of 3-Fluorooxindole Derivatives Using Diethyl 2-Fluoromalonate
Durham E-Theses Synthesis of 3-uoro-oxindoles and phenyl uoroacetic acid derivatives HARSANYI, ANTAL How to cite: HARSANYI, ANTAL (2013) Synthesis of 3-uoro-oxindoles and phenyl uoroacetic acid derivatives, Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/6357/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk A Thesis Entitled Synthesis of 3-fluoro-oxindoles and phenyl fluoroacetic acid derivatives Submitted by Antal Harsanyi BSc (College of St. Hild and St. Bede) Department of Chemistry A Candidate for the Degree of Master of Science 2012 Declaration The work presented within this thesis was carried out at Durham University between October 2011 and August 2012. This thesis is the work of the author, except where acknowledged by reference and has not been submitted for any other degree. Part of this work has been presented at: 12th RSC Annual Fluorine Group Meeting, St Andrews, August 2012 Statement of copyright The copyright of this thesis rests with the author. -

The Development and Application of Metal-Catalyzed Processes for Organic Synthesis
The Development and Application of Metal-Catalyzed Processes for Organic Synthesis by Edward J. Hennessy B.A. Chemistry Northwestern University, 2000 Submitted to the Department of Chemistry in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY IN ORGANIC CHEMISTRY at the Massachusetts Institute of Technology June 2005 © 2005 Massachusetts Institute of Technology All Rights Reserved Signature of Author: " - - 1_"' .Da-uirnt of Chemistry May 19, 2005 Certified by: Stephen L. Buchwald Camille Dreyfus Professor of Chemistry Thesis Supervisor Accepted by: Robert W. Field Chairman, Departmental Committee on Graduate Students ARCHIVES: This doctoral thesis has been examined by a committee of the Department of Chemistry as follows: -/ Professor Gregory C. Fu: " Ch Chair Professor Stephen L. Buchwald Thesis Supervisor Professor Timothy M. Swager: , -L i \ O 2 The Development and Application of Metal-Catalyzed Processes for Organic Synthesis by Edward J. Hennessy Submitted to the Department of Chemistry on May 19, 2005 in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy at the Massachusetts Institute of Technology ABSTRACT Chapter 1. Copper-Catalyzed Arylation of Stabilized Carbanions A mild, general catalytic system for the synthesis of a-aryl malonates has been developed. Aryl iodides bearing a variety of functional groups can be effectively coupled to diethyl malonate in high yields using inexpensive and widely available reagents, making this a superior method to those previously described that employ copper reagents or catalysts. The functional group tolerance of the process developed makes it complementary to analogous palladium-catalyzed couplings. Importantly, a set of mild reaction conditions has been developed that minimize product decomposition, a problem that had not been addressed previously in the literature. -

Design, Synthesis, and Supramolecular Surface Chemistry Of
DESIGN, SYNTHESIS, AND SUPRAMOLECULAR SURFACE CHEMISTRY OF BI- AND TRIDENTATE SURFACE ANCHORS FOR NANOSCIENCE AND NANOBIOTECHNOLOGY A Dissertation Presented to The Graduate Faculty of the University of Akron In Partial Fulfillment Of the Requirements for the Degree Doctor of Philosophy Hui Wang August, 2007 DESIGN, SYNTHESIS, AND SUPRAMOLECULAR SURFACE CHEMISTRY OF BI- AND TRIDENTATE SURFACE ANCHORS FOR NANOSCIENCE AND NANOBIOTECHNOLOGY Hui Wang Dissertation Approved: Accepted: _______________________ _______________________ Advisor Department Chair Dr. Jun Hu Dr. Kim C. Calvo _______________________ _______________________ Committee Member Dean of the College Dr. Gerald F. Koser Dr. Ronald F. Levant _______________________ _______________________ Committee Member Dean of the Graduate School Dr. David A. Modarelli Dr. George R. Newkome _______________________ _______________________ Committee Member Date Dr. Claire A. Tessier _______________________ Committee Member Dr. Robert R. Mallik ii ABSTRACT This dissertation describes the design, synthesis, and supramolecular surface chemistry of bi- and tri-dentate surface anchors for nanoscience and nanobiotechnology. Molecular electronic device candidates, based on the tridentate surface anchor 2,4,9-trithia-tricyclo[3.3.1.13,7]decane, were used to bridge two ruthenium metal clusters. These well-designed ruthenium complexes were used as nanometer-sized molecular connector/metal cluster models to investigate the surface binding characteristics of tridentate surface anchor-metal junctions. Bi-dentate surface anchors, 1,4-dimercapto-2,3-dimethyl-butane- 2,3-diol and 4,5-dimethyl-2-(4-vinyl-phenyl)-[1,3,2]dioxaborolane-4,5-dithiol, were synthesized. They were used as ligands for stabilizing gold nanoparticles by two methods: direct reduction reaction, and ligand exchange reaction with triphenylphosphine-stabilized gold nanoparticles. -

FAROOK COLLEGE (Autonomous)
FAROOK COLLEGE (Autonomous) M.Sc. DEGREE PROGRAMME IN CHEMISTRY CHOICE BASED CREDIT AND SEMESTER SYSTEM-PG (FCCBCSSPG-2019) SCHEME AND SYLLABI 2019 ADMISSION ONWARDS 1 CERTIFICATE I hereby certify that the documents attached are the bona fide copies of the syllabus of M.Sc. Chemistry Programme to be effective from the academic year 2019-20 onwards. Date: Place: P R I N C I P A L 2 FAROOK COLLEGE (AUTONOMOUS) MSc. CHEMISTRY (CSS PATTERN) Regulations and Syllabus with effect from 2019 admission Pattern of the Programme a. The name of the programme shall be M.Sc. Chemistry under CSS pattern. b. The programme shall be offered in four semesters within a period of two academic years. c. Eligibility for admission will be as per the rules laid down by the College from time to time. d. Details of the courses offered for the programme are given in Table 1. The programme shall be conducted in accordance with the programme pattern, scheme of examination and syllabus prescribed. Of the 25 hours per week, 13 hours shall be allotted for theory and 12 hours for practical; 1 theory hour per week during even semesters shall be allotted for seminar. Theory Courses In the first three semesters, there will be four theory courses; and in the fourth semester, three theory courses. All the theory courses in the first and second semesters are core courses. In the third semester there will be three core theory courses and one elective theory course. College can choose any one of the elective courses given in Table 1. -

Study on Accurate Determination of Volatile Fatty Acids in Rumen Fluid
5th International Conference on Information Engineering for Mechanics and Materials (ICIMM 2015) Study on Accurate Determination of Volatile Fatty Acids in Rumen Fluid by Capillary Gas Chromatography Cheng LUOa, Suyi CAI, Linyan JIA, Xiang TANG, Ruinan ZHANG, Gang JIA, Hua LI, Jiayong TANG, Guangmang LIU, Caimei WU*b Institute of Animal Nutrition, Key Laboratory for Animal Disease-Resistance Nutrition of Sichuan Province, Key Laboratory for Animal Disease-Resistance Nutrition and Feedstuffs of China Ministry of Agriculture, Sichuan Agricultural University, cheng’du 611130, China aemail: [email protected], *bemail:[email protected] Key Words: Capillary gas chromatography; Rumen fluid; Volatile fatty acids; Internal standard method Abstract. In order to obtain accurate results of volatile fatty acids (VFAs) in rumen fluid by capillary gas chromatography (GC), a pretreatment method was firstly conducted by dissolving VFAs in the methanol. An internal standard method (ISM) with crotonic acid as the internal standard was adopted in the detection. The results showed that the reproducibility of VFA (acetic acid, propanoic acid and butyric acid) was enhanced remarkably with methanol added as the solvent. The reproducibility was dependent on both of the VFA concentration and methanol amount. In conclusion, methanol solvent was more suitable than aqueous solvent for VFA accurate detection with ISM by capillary GC. Introduction Volatile fatty acids (VFAs) got through rumen fermentation were important energy sources for ruminant animals [1]. The acetic acid, propanoic acid, butyric acid accounted for about 95% of the total VFAs. Therefore, it was significant to determine acetic acid, propanoic acid and butyric accurately for the rumen fermentation study. In recent years, acetic acid, propanoic acid and butyric acid were detected by GC with packed column or capillary column [1-12]and ion chromatography (IC) [13]. -

Organometallic Compounds
Chapter 11 Organometallic compounds Organometallics Reactions using organometallics Organometallic compounds Ch 11 #2 = comp’s containing a carbon-metal bond δ− δ+ C in organometallic comp’ds are nucleophilic. C in organic comp’d (like ROH, RNH , and RX) 2 δ+ δ are electrophilic. − due to ∆EN Ch 11 #3 in substitution reactions a carbon Nu: R-Li and R-MgX Ch 11 #4 (used to be) the two most common organometallics organolithium comp’ds BuLi = an alkyl lithium organomagnesium comp’ds = Grignard reagents 1912 Nobel Prize R, Ar, vinyl all possible; Br (as X) popular Ether (solvent) coordinates Mg, stabilizing it. Reactions of R-Li and R-MgX Ch 11 #5 reacts like a carbanion C:– ~ a C Nu: reactions as C Nu: (like SN(2)) nucleophilic addition to carbonyls ~ more often Chapt 16 Ch 11 #6 R-Li and R-MgX are very strong B: how strong? pKa? − − react even with very weak acid stronger than OH? NH2? useful for preparing deuterated HC Storage and reaction must be acid- and moisture-free. Transmetal(l)ation Ch 11 #7 R-Li is more reactive than R-MgX is. C–Li more polar than C–Mg C of R-Li more nu-philic [better Nu:] transmetalation [metal exchange] to less reactive [more stable] organometallic Coupling using Gilman reagent Ch 11 #8 coupling reaction (in organic chemistry) two hydrocarbon fragments are coupled (to form C–C) with the aid of a (transition) metal catalyst Gilman reagent coupling of R of R-X and R’ of Gilman reagent RX + R’2CuLi R–R’ mechanism? substitution of X with R’? not clear Ch 11 #9 R can be alkyl, aryl, or alkenyl [vinyl] which is not possible by R-Li or R-MgX Is R of Gilman why? they are SN(2). -
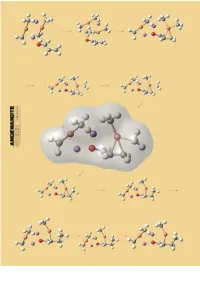
Structures and Reaction Mechanisms of Organocuprate Clusters in Organic Chemistry
REVIEWS Wherefore Art Thou Copper? Structures and Reaction Mechanisms of Organocuprate Clusters in Organic Chemistry Eiichi Nakamura* and Seiji Mori Organocopper reagents provide the principles. This review will summarize example of molecular recognition and most general synthetic tools in organic first the general structural features of supramolecular chemistry, which chemistry for nucleophilic delivery of organocopper compounds and the pre- chemists have long exploited without hard carbanions to electrophilic car- vious mechanistic arguments, and then knowing it. Reasoning about the bon centers. A number of structural describe the most recent mechanistic uniqueness of the copper atom among and mechanistic studies have been pictures obtained through high-level neighboring metal elements in the reported and have led to a wide variety quantum mechanical calculations for periodic table will be presented. of mechanistic proposals, some of three typical organocuprate reactions, which might even be contradictory to carbocupration, conjugate addition, Keywords: catalysis ´ conjugate addi- others. With the recent advent of and SN2 alkylation. The unified view tions ´ copper ´ density functional physical and theoretical methodolo- on the nucleophilic reactivities of met- calculations ´ supramolecular chemis- gies, the accumulated knowledge on al organocuprate clusters thus ob- try organocopper chemistry is being put tained has indicated that organocup- together into a few major mechanistic rate chemistry represents an intricate 1. Introduction 1 R Cu X The desire to learn about the nature of elements has been R or R1 R and will remain a main concern of chemists. In this review, we R Cu will consider what properties of copper make organocopper R1 chemistry so useful in organic chemistry. -

Acetoacetic Ester Synthesis
Programme: B.Sc. B.ed. (Integrated) Course: ORGANIC CHEMISTRY- III Semester: VI Code: CHE-352 Topic: ETHYLACETOACETATEE Date- 07/04/2020 y Only PPe Dr. Angad Kumar Singh ForF Department of Chemistry, Central University of South Bihar, Gaya (Bihar) Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data taken from several books, research articles including Wikipedia. Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data taken from several books, research articles including Wikipedia. Ethylacetoacetate The Claisen Condensation between esters containing - hdhydrogens, promotdted byabase such as sodium ethoxy ide, toproduce a !- ketoester. One equiv serves as the nucleophilele (enolate)(eno and the other is OnlyO the electrophile which undergoes additiontion andae elimination. The use of stronger bases, e.g. sodium amide or sodiumsodUse hydride instead of sodium ethoxide, often increases the yield.d. al onal Ethylacetoacetate Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data taken from several books, research articles including Wikipedia. Mechanism of the Claisen Condensation The reaction is driven to product by the final deprotonation step. Note: These materials are only for classroom teaching purpose at Central University of South Bihar. All the data taken from several books, research articles including Wikipedia. Mixed Claisen Condensation Like mixed aldol reactions, mixed Claisen condensations are useful if differences in reactivity exist betweenn they two esters as for example when one of the esters has no -hydrogenydrogOnlyO . Examples of such esters are: e Use ers excess Note: These materials are only for classroom teaching purpose at Central University of South Bihar. -

Diethyl Malonate
DIETHYL MALONATE PRODUCT IDENTIFICATION CAS NO. 105-53-3 EINECS NO. 203-305-9 FORMULA CH 2(COOC 2H5)2 MOL WT. 160.17 H.S. CODE TOXICITY SYNONYMS Ethyl methane dicarboxylate; Ethyl propanedioate; Diethylmalonat (German); Malonato de dietilo (Spanish); Malonate de diéthyle (French); Ethyl malonate; Malonic; Propanedioic acid diethyl ester; DERIVATION CLASSIFICATION PHYSICAL AND CHEMICAL PROPERTIES PHYSICAL STATE clear liquid MELTING POINT -50 C BOILING POINT 199 C SPECIFIC GRAVITY 1.055 SOLUBILITY IN WATER Slightly soluble pH VAPOR DENSITY 5.5 AUTOIGNITION NFPA RATINGS Health: 0; Flammability: 1; Reactivity: 0 REFRACTIVE INDEX 1.4135 FLASH POINT 93 C STABILITY Stable under ordinary conditions GENERAL DESCRIPTION & APPLICATIONS Malonic acid (also called Propanedioic Acid ) is a white crystalline C-3 dicarboxylic acid; melting at 135 -136 C; readily soluble in water, alcohol and ether; solution in water is medium-strong acidic. It can be derived by oxidizing malic acid or by the hydrolysis of cyanacetic acid. Malonic acid itself is rather unstable and has few applications. It's diethyl ester (diethyl malonate) is more important commercially. Diethyl malonate, a colourless, fragrant liquid boiling at 199 C, is prepared by the reaction of monochloroacetatic acid with methanol, carbon monoxide or by the reaction cyanoacetic acid (the half nitriled-malonic acid) with ethyl alcohol. Malonic acid and its esters contain active methylene groups which have relatively acidic alpha-protons due to H atoms adjacent to two carbonyl groups. The reactivity of its methylene group provide the sequence of reactions of alkylation, hydrolysis of the esters and decarboxylation resulting in substituted ketones. The methylene groups in 1,3-dicarboxylic acid utilize the synthesis of barbiturates; a hydrogen atom is removed by sodium ethoxide, and the derivative reacts with an alkyl halide to form a diethyl alkylmalonate. -

Ethyl Acetoacetate
21.6 The Acetoacetic Ester Synthesis Acetoacetic Ester O O C C H3C C OCH2CH3 H H Acetoacetic ester is another name for ethyl acetoacetate. The "acetoacetic ester synthesis" uses acetoacetic ester as a reactant for the preparation of ketones. Deprotonation of Ethyl Acetoacetate O O – C C + CH3CH2O H3C C OCH2CH3 H H Ethyl acetoacetate pKa ~ 11 can be converted readily to its anion with bases such as sodium ethoxide. Deprotonation of Ethyl Acetoacetate O O – C C + CH3CH2O H3C C OCH2CH3 H H Ethyl acetoacetate pKa ~ 11 can be converted readily to its anion K ~ 105 K ~ 10 with bases such as O O sodium ethoxide. C •• C + CH3CH2OH H3C –C OCH2CH3 3 2 H pKa ~ 16 Alkylation of Ethyl Acetoacetate O O The anion of ethyl C •• C acetoacetate can be H C C OCH CH 3 – C OCH2CH3 alkylated using an H alkyl halide (SN2: primary and R X secondary alkyl halides work best; tertiary alkyl halides undergo elimination). Alkylation of Ethyl Acetoacetate O O The anion of ethyl C •• C acetoacetate can be H C C OCH CH 3 – C OCH2CH3 alkylated using an H alkyl halide (SN2: primary and R X secondary alkyl O O halides work best; tertiary alkyl halides C C undergo elimination). H3C C OCH2CH3 H R Conversion to Ketone O O Saponification and C C acidification convert H3C C OH the alkylated H R derivative to the – 1. HO , H2O corresponding b-keto 2. H+ acid. O O The b-keto acid then undergoes C C decarboxylation to H C 3 C OCH2CH3 form a ketone. -

The Addition of Bromine to Crotonic Acid and to Ethyl Crotonate Under Various Conditions
Proceedings of the Iowa Academy of Science Volume 59 Annual Issue Article 21 1952 The Addition of Bromine to Crotonic Acid and to Ethyl Crotonate under Various Conditions Robert E. Buckles State University of Iowa Arne Langsjoen State University of Iowa Rex E. Selk State University of Iowa Robert J. Coons State University of Iowa Let us know how access to this document benefits ouy Copyright ©1952 Iowa Academy of Science, Inc. Follow this and additional works at: https://scholarworks.uni.edu/pias Recommended Citation Buckles, Robert E.; Langsjoen, Arne; Selk, Rex E.; and Coons, Robert J. (1952) "The Addition of Bromine to Crotonic Acid and to Ethyl Crotonate under Various Conditions," Proceedings of the Iowa Academy of Science, 59(1), 170-177. Available at: https://scholarworks.uni.edu/pias/vol59/iss1/21 This Research is brought to you for free and open access by the Iowa Academy of Science at UNI ScholarWorks. It has been accepted for inclusion in Proceedings of the Iowa Academy of Science by an authorized editor of UNI ScholarWorks. For more information, please contact [email protected]. Buckles et al.: The Addition of Bromine to Crotonic Acid and to Ethyl Crotonate u The Addition of Bromine to Crotonic Acid and to Ethyl Crotonate under Various Conditions By ROBERT E. BUCKLES, ARNE LANGSJOEN, REX E. SELK, AND ROBERT J. COONS Addition of bromine to ethyl crotonate in the absence of solvent has been reported ( 1) to give a high yield of ethyl a, ,B-dibromo butyrate. This result was verified in the present work. Neither ethyl crotonate nor crotonic acid, however, gave a positive test with bromine in carbon tetrachloride at room temperature.