Influence Ofapplication Rate and Timing Ofdiclosulam on Weed Control in Peanut (Arachis Hypogaea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tolerance of Virginia-Type Peanut to Different Application Timings of 2,4-DB
Tolerance ofVirginia-Type Peanut to Different Application Timings of 2,4-DB T. A. Baughman*, W. J. Grichar, and D. L. [ordan' ABSTRACT ductive periods. Astudyconductedin Texas on spanish-type Field studies were conducted to determine the ef peanut indicated 2,4-DB applied between maximum peg fects of2,4-DB application timings on yield and market ging and early pod (fruit) enlargement reduced yield and qualityofvirginia-typepeanut. Trialswere conducted at affected quality and pod size (Ketchersid et al., 1978). three locations in Texasand one location in North Caro However, theseyield reductions occurredwhen2,4-DB was linain 1997,1998, and 1999. 2,4-DB at 0.45 kgaelhawas applied at 0.9 kglha, which is more than the registered rate. applied 30, 45, 60, 90, and 120 d after planting (DAP). Multiple applications at 0.45 kglha did not affect spanish Additional timings included combinations of 30 DAP peanut (Ketchersid et al., 1978). Grichar et al. (1997) followedby (fb) 60, 90, or 120 DAP;60 DAP fb 90 or 120 reported that single and multiple applications of 2,4-DB at DAP; and 90 DAP fb 120 DAP. Peanut yield, market 0.45 kglha did not affect runner-type peanutyield or market grade factors, and pod and seed weight were not influ grade characteristics. enced by various application timings of 2,4-DB. Limiteddataare available documentingthe effect of 2,4 DB on virginia-type peanut yield and grades when applied at various times throughoutthe growing season. Jordanet al. Key Words: Crop tolerance, market grade, yield. (2001) suggested that late season applications of2,4-DB did not affect podyield or market grade characteristics. -

Species List For: Labarque Creek CA 750 Species Jefferson County Date Participants Location 4/19/2006 Nels Holmberg Plant Survey
Species List for: LaBarque Creek CA 750 Species Jefferson County Date Participants Location 4/19/2006 Nels Holmberg Plant Survey 5/15/2006 Nels Holmberg Plant Survey 5/16/2006 Nels Holmberg, George Yatskievych, and Rex Plant Survey Hill 5/22/2006 Nels Holmberg and WGNSS Botany Group Plant Survey 5/6/2006 Nels Holmberg Plant Survey Multiple Visits Nels Holmberg, John Atwood and Others LaBarque Creek Watershed - Bryophytes Bryophte List compiled by Nels Holmberg Multiple Visits Nels Holmberg and Many WGNSS and MONPS LaBarque Creek Watershed - Vascular Plants visits from 2005 to 2016 Vascular Plant List compiled by Nels Holmberg Species Name (Synonym) Common Name Family COFC COFW Acalypha monococca (A. gracilescens var. monococca) one-seeded mercury Euphorbiaceae 3 5 Acalypha rhomboidea rhombic copperleaf Euphorbiaceae 1 3 Acalypha virginica Virginia copperleaf Euphorbiaceae 2 3 Acer negundo var. undetermined box elder Sapindaceae 1 0 Acer rubrum var. undetermined red maple Sapindaceae 5 0 Acer saccharinum silver maple Sapindaceae 2 -3 Acer saccharum var. undetermined sugar maple Sapindaceae 5 3 Achillea millefolium yarrow Asteraceae/Anthemideae 1 3 Actaea pachypoda white baneberry Ranunculaceae 8 5 Adiantum pedatum var. pedatum northern maidenhair fern Pteridaceae Fern/Ally 6 1 Agalinis gattingeri (Gerardia) rough-stemmed gerardia Orobanchaceae 7 5 Agalinis tenuifolia (Gerardia, A. tenuifolia var. common gerardia Orobanchaceae 4 -3 macrophylla) Ageratina altissima var. altissima (Eupatorium rugosum) white snakeroot Asteraceae/Eupatorieae 2 3 Agrimonia parviflora swamp agrimony Rosaceae 5 -1 Agrimonia pubescens downy agrimony Rosaceae 4 5 Agrimonia rostellata woodland agrimony Rosaceae 4 3 Agrostis elliottiana awned bent grass Poaceae/Aveneae 3 5 * Agrostis gigantea redtop Poaceae/Aveneae 0 -3 Agrostis perennans upland bent Poaceae/Aveneae 3 1 Allium canadense var. -

Weed Science
Integrated Pest Management MISSOURI Plant Protection Programs College of Agriculture, Food and Natural Resources Published by MU Extension, University of Missouri-Columbia $5.00 IPM1023 This publication is part of a series of IPM ManualsManuals prepared byby the Plant Protection ProgramsPrograms of the UniversityUniversity of Missouri.Missouri. TopicsTopics coveredcovered in the seriesseries include an introduction to scouting, weedweed identifi cation and management, plant diseases,diseases, and insects of fi eld and horticulturalhorticultural crops.crops. These IPM ManualsManuals are availableavailable from MU Extension at the following address: Extension Publications 2800 Maguire Blvd. Columbia, MO 65211 1-800-292-0969 CONTENTS Authors Broadleaf plant families. 4 Fred Fishel Department of Agronomy Common name index - Broadleaf plant Universiity of Missouri-Columbia families. 16 Kevin Bradley Grass and grasslike plant families. 17 Department of Agronomy Common name index - Grass and grasslike University of Missouri-Columbia plant families . 19 On the World Wide Web For this and other Integrated Pest Management publications on the World Wide Web, visit http://ipm.missouri.edu. Production MU Extension and Agricultural Information Tammy McNiel, editor Dennis Murphy, illustrator © 2005 University of Missouri A PHOTO COMPENDIUM OF MISSOURI WEED SEEDS ositive identifi cation of pests, includ- ruler. The distance between increments on the ing weeds, is the fi rst step in a sound sacle is 1 mm. Compare your sample with the Pintegrated pest management program. color images in this guide to assist in your iden- Knowledge of plant morphological features, tifi cation. Seed photographs are grouped by such as leaf and stem shape, fl ower type and their plant taxonomic family for both broadleaf color, and the presence of hairs make identi- and grass or grasslike weeds. -
![Vascular Plants of Williamson County Croton Glandulosus Var. Lindheimeri − LINDHEIMER’S TROPIC CROTON [Euphorbiaceae]](https://docslib.b-cdn.net/cover/5594/vascular-plants-of-williamson-county-croton-glandulosus-var-lindheimeri-lindheimer-s-tropic-croton-euphorbiaceae-1385594.webp)
Vascular Plants of Williamson County Croton Glandulosus Var. Lindheimeri − LINDHEIMER’S TROPIC CROTON [Euphorbiaceae]
Vascular Plants of Williamson County Croton glandulosus var. lindheimeri − LINDHEIMER’S TROPIC CROTON [Euphorbiaceae] Croton glandulosus L. var. lindheimeri Muell.-Arg, LINDHEIMER’S TROPIC CROTON. Annual, taprooted, 1-stemmed at base, not rosetted, with an ascending to spreading branch on principal axis from each node, 10−22 cm tall; monoecious; shoots with only cauline leaves, densely stellate-pubescent with arms of hair unequal. Stems: cylindric, to 2.5 mm diameter, tough, green aging with light brown periderm on lower plant, having stellate hairs persistent on periderm. Leaves: helically alternate, simple, petiolate, with stipules; stipules 2, attached to stem at node, fingerlike, 1−1.5 mm long, with several stellate hairs and having some erect, 1-armed hairs at tip; petiole channeled, < 8−20 mm long, < blade, projecting from the edges at top of petiole and blade junction with a pair of glands (extrafloral nectaries), the nectaries cuplike and ± 0.6 mm across, in nature with a drop of exudate; blade oblong-narrowly ovate to ovate, 12−45 × 10−22 mm, tapered at base, serrate on margins, toothed to subtruncate at tip, pinnately veind with 3 veins at base and principal veins raised on lower surface, upper surface having hairs with fewer arms (some 1-armed), lower surface stellate-hairy throughout, arms subequal but with erect central arm. Inflorescence: raceme, with 1−3 pistillate flowers at base and < 10 staminate flowers above, bracteate, stellate-pubescent; raceme green, near base with 2 stalked, orangish yellow glands 0.35−0.4 mm long; bractlet subtending pedicel of staminate flowers club-shaped in outline, 0.5−0.7 mm long, < pedicel, ± green. -

7 Artigo Croton.Indd
O gênero Croton L. (Euphorbiaceae s.s. – Crotonoideae) na Floresta Nacional de Silvânia, Goiás, Brasil 89 O gênero Croton L. ( Euphorbiaceae s.s. – Crotonoideae ) na Floresta Nacional de Silvânia, Goiás, Brasil Rodolfo Carneiro Sodré & Marcos José da Silva Universidade Federal de Goiás, Instituto de Ciências Biológicas, Departamento de Botânica. Alameda Ingá, Quadra A, Campus Samambaia, CEP 74001-970, Goiânia, GO, Brasil. [email protected], [email protected] Recebido em 11.VIII.2014. Aceito em 20.V.2015. RESUMO – Como parte do inventário das espécies de Croton L. no estado de Goiás apresentamos o levantamento taxonômico deste gênero na Floresta Nacional de Silvânia (FLONA-Silvânia). Foram reconhecidas dez espécies: C. abaitensis Baill., C. agrarius Baill., C. antisyphiliticus Mart., C. glandulosus L., C . goyazensis Müll. Arg., C. intercedens Müll. Arg., C. sclerocalyx (Didr.) Müll. Arg., C. spica Baill., C. tamberlikii Müll. Arg. e C. urucurana Baill., diferenciadas principalmente pela forma das glândulas foliares e morfologia das fl ores pistiladas. Croton abaitensis, C. spica e C. tamberlikii são novas ocorrências para Goiás e as duas primeiras, juntamente com C. agrarius e C. intercedens, são aqui primeiramente ilustradas. São apresentadas chave de identifi cação, descrições e ilustrações dos táxons, bem como comentários sobre suas distribuições, habitats e relações morfológicas. Palavras-chave: Brasil Central, Cerrado, Crotoneae, taxonomia ABSTRACT – The genus Croton (Euphorbiaceae s.s. – Crotonoideae ) in the National Forest of Silvânia, Goiás, Brazil. A taxonomic survey of the genus Croton in The National Forest of Silvânia (FLONA-Silvânia) is presented as a part of the inventory of this genus in the state of Goiás. Ten species were recognized: C. -

Woody and Herbaceous Plants Native to Haiti for Use in Miami-Dade Landscapes1
Woody and Herbaceous Plants Native to Haiti For use in Miami-Dade Landscapes1 Haiti occupies the western one third of the island of Hispaniola with the Dominican Republic the remainder. Of all the islands within the Caribbean basin Hispaniola possesses the most varied flora after that of Cuba. The plants contained in this review have been recorded as native to Haiti, though some may now have been extirpated due in large part to severe deforestation. Less than 1.5% of the country’s original tree-cover remains. Haiti’s future is critically tied to re- forestation; loss of tree cover has been so profound that exotic fast growing trees, rather than native species, are being used to halt soil erosion and lessen the risk of mudslides. For more information concerning Haiti’s ecological plight consult references at the end of this document. For present purposes all of the trees listed below are native to Haiti, which is why non-natives such as mango (the most widely planted tree) and other important trees such as citrus, kassod tree (Senna siamea) and lead tree (Leucanea leucocephala) are not included. The latter two trees are among the fast growing species used for re-forestation. The Smithsonian National Museum of Natural History’s Flora of the West Indies was an invaluable tool in assessing the range of plants native to Haiti. Not surprisingly many of the listed trees and shrubs 1 John McLaughlin Ph.D. U.F./Miami-Dade County Extension Office, Homestead, FL 33030 Page | 1 are found in other parts of the Caribbean with some also native to South Florida. -

Supplemental Label
Supplemental Label EPA Reg. No. 59639-99 (For Use Only in Idaho, Oregon and Washington) VALOR® SX HERBICIDE USE ON CHICKPEA (GARBANZO BEAN) DIRECTIONS FOR USE It is a violation of Federal law to use this product in a manner inconsistent with its labeling. THIS LABELING MUST BE IN THE POSSESSION OF THE USER AT THE TIME OF APPLICATION. READ THE LABEL AFFIXED TO THE CONTAINER FOR VALOR HERBICIDE BEFORE APPLYING. USE OF VALOR HERBICIDE ACCORDING TO THIS LABELING IS SUBJECT TO THE USE PRECAUTIONS AND LIMITATIONS IMPOSED BY THE LABEL AFFIXED TO THE CONTAINER FOR VALOR HERBICIDE. RESTRICTIONS AND LIMITATIONS Do not apply more than 2.0 oz of Valor Herbicide per acre during a single application. Do not apply more than 2.0 oz of Valor Herbicide per acre during a single growing season. Many weather related factors, including high wind, splashing or heavy rains or cool conditions at or near crop emergence, may result in garbanzo bean injury in fields treated with Valor Herbicide. On occasion this has resulted in a delay in maturity. User should assume these risks before using Valor Herbicide. TIMING TO CHICKPEA (GARBANZO BEAN) Valor Herbicide may be applied to garbanzo beans within 2 days after planting for the preemergence suppression of the weeds listed in Table A, Broadleaf Weeds Controlled by Residual Activity of Valor Herbicide. Tank mix Valor Herbicide with other labeled herbicides for broad spectrum weed control. TIMING TO WEEDS Valor Herbicide may be applied to garbanzo beans prior to planting or preemergence (after planting). Preemergence application of Valor Herbicide must be made within 2 days after planting and prior to garbanzo bean emergence. -

Glyphosate-Resistant Soybean Interference in Glyphosate-Resistant Cotton Donna R
The Journal of Cotton Science 13:178–182 (2009) 178 http://journal.cotton.org, © The Cotton Foundation 2009 WEED SCIENCE Glyphosate-Resistant Soybean Interference in Glyphosate-Resistant Cotton Donna R. Lee1, Donnie K. Miller2, David C. Blouin3, Scott B. Clewis4 and Wesley J. Everman5 ABSTRACT and Wilcut, 2007; Culpepper and York, 1999). In addition, glyphosate-based management systems A negative aspect of glyphosate-based man- in cotton allow producers to integrate weed, insect, agement systems is the potential for volunteer and crop management strategies through chemical glyphosate-resistant (GR) crop plants to impact co-application of glyphosate with insecticides, subsequent crops. Studies were conducted to micronutrients, and plant growth regulators (Scroggs evaluate density and duration of interference ef- et al., 2005; Miller et al., 2008). Due in part to fects of GR soybean (Glycine max (L.) Merr.) on these positive attributes, GR cotton has been widely GR cotton (Gossypium hirsutum L.) growth and accepted by growers, with 74% of the crop in the yield. Our study demonstrated that regardless United States planted to GR cultivars less than of soybean density and duration of interference, a decade after commercialization (Sankula and soybean did not affect cotton height at harvest. Blumenthal, 2004). Season-long interference with a soybean density One negative aspect of glyphosate-based man- of 1 plant per row m would be expected to reduce agement systems in GR cultivars is the potential cotton yield 14%. A cotton yield reduction of 0.4 for volunteer GR crop plants in subsequent crops, to 1% can be expected with only 1 week of soy- thereby requiring additional management inputs bean interference at a density of 5.25 plants per (York et al., 2004, 2005). -

Lepidoptera: Gracillariidae): an Adventive Herbivore of Chinese Tallowtree (Malpighiales: Euphorbiaceae) J
Host range of Caloptilia triadicae (Lepidoptera: Gracillariidae): an adventive herbivore of Chinese tallowtree (Malpighiales: Euphorbiaceae) J. G. Duncan1, M. S. Steininger1, S. A. Wright1, G. S. Wheeler2,* Chinese tallowtree, Triadica sebifera (L.) Small (Malpighiales: Eu- and the defoliating mothGadirtha fusca Pogue (Lepidoptera: Nolidae), phorbiaceae), native to China, is one of the most aggressive and wide- both being tested in quarantine to determine suitability for biological spread invasive weeds in temperate forests and marshlands of the control (Huang et al. 2011; Wang et al. 2012b; Pogue 2014). The com- southeastern USA (Bruce et al. 1997). Chinese tallowtree (hereafter patibility of these potential agents with one another and other herbi- “tallow”) was estimated to cover nearly 185,000 ha of southern for- vores like C. triadicae is being examined. The goal of this study was to ests (Invasive.org 2015). Since its introduction, the weed has been re- determine if C. triadicae posed a threat to other native or ornamental ported primarily in 10 states including North Carolina, South Carolina, plants of the southeastern USA. Georgia, Florida, Alabama, Mississippi, Louisiana, Arkansas, Texas, and Plants. Tallow plant material was field collected as seeds, seed- California (EddMapS 2015). Tallow is now a prohibited noxious weed lings, or small plants in Alachua County, Florida, and cultured as pot- in Florida, Louisiana, Mississippi, and Texas (USDA/NRCS 2015). As the ted plants and maintained in a secure area at the Florida Department existing range of tallow is expected to increase, the projected timber of Agriculture and Consumer Services, Division of Plant Industry. Ad- loss, survey, and control costs will also increase. -
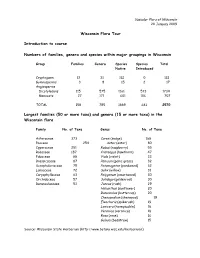
Wisconsin Flora Tour Introduction to Course Numbers of Families, Genera
Vascular Flora of Wisconsin 20 January 2009 Wisconsin Flora Tour Introduction to course Numbers of families, genera and species within major groupings in Wisconsin Group Families Genera Species Species Total Native Introduced Cryptogams 13 31 112 0 112 Gymnosperms 3 8 15 2 17 Angiosperms Dicotyledons 115 575 1161 573 1734 Monocots 27 171 601 106 707 TOTAL 158 785 1889 681 2570 Largest families (50 or more taxa) and genera (15 or more taxa) in the Wisconsin flora Family No. of Taxa Genus No. of Taxa Asteraceae 373 Carex (sedge) 168 Poaceae 254 Aster (aster) 80 Cyperaceae 251 Rubus (raspberry) 55 Rosaceae 187 Crateagus (hawthorn) 47 Fabaceae 88 Viola (violet) 33 Brassicaceae 87 Panicum (panic grass) 32 Scrophulariaceae 75 Potamogeton (pondweed) 32 Lamiaceae 72 Salix (willow) 31 Caryophyllaceae 63 Polygonum (smartweed) 30 Orchidaceae 57 Solidago (goldenrod) 30 Ranunculaceaee 53 Juncus (rush) 29 Helianthus (sunflower) 20 Ranunculus (buttercup) 20 Chenopodium (chenopod) 19 Eleocharis (spikerush) 19 Lonicera (honeysuckle) 18 Veronica (veronica) 18 Rosa (rose) 16 Galium (bedstraw) 15 Source: Wisconsin State Herbarium (http://www.botany.wisc.edu/herbarium/) Four major floristic elements in the Wisconsin flora Boreal Alleghenian Ozarkian Prairie Two floristic provinces Northern hardwood Prairie forests Tension Zone Brief look at four plant communities Beech maple or southern mesic Oak forest or southern xeric Prairie Bog or fen Vascular Flora of Wisconsin 22 January 2009 Nomenclature and Vascular Cryptogams I Nomenclature vs. Classification Rank -

A Comparison of Native Versus Old-Field Vegetation in Upland Pinelands Managed with Frequent Fire, South Georgia, Usa
A COMPARISON OF NATIVE VERSUS OLD-FIELD VEGETATION IN UPLAND PINELANDS MANAGED WITH FREQUENT FIRE, SOUTH GEORGIA, USA Thomas E. Ostertag1 and Kevin M. Robertson2 Tall Timbers Research Station, 13093 Henry Beadel Drive, Tallahassee, FL 32312, USA ABSTRACT Fire-maintained, herb-dominated upland pinelands of the southeastern U.S. Coastal Plain may be broadly divided into those that have arisen through secondary succession following abandonment of agriculture (old-field pinelands) and those that have never been plowed (native pinelands). The ability to distinguish these habitat types is important for setting conservation priorities by identifying natural areas for conservation and appropriate management and for assessing the ecological value and restoration potential for old-field pine forests managed with frequent fire. However, differences in species composition have rarely been quantified. The goals of this study were to characterize the species composition of native and old-field pineland ground cover, test the ability to distinguish communities of previously unknown disturbance history, and suggest indicator species for native versus old-field pinelands. Plant composition was surveyed in areas known to be native ground cover, those known to be old fields, and those with an uncertain disturbance history. Twelve permanent plots were established in each cover type and sampled in spring (April–May) and fall (October–November) in 2004 and 2005. Of the 232 species identified in the plots, 56 species were present only in native ground-cover plots, of which 17 species occurred in a sufficient number of plots to have a statistically significant binomial probability of occurring in native ground cover and might be considered indicator species. -

Pasture Weed Control in Arkansas
Pasture Weed Control in Arkansas DIVISION OF AGRICULTURE RESEARCH & EXTENSION MP522 University of Arkansas System 2 Pasture Weed Control in Arkansas By: John Boyd, Ph.D. Blair Griffin Visiting Assistant Professor County Extension Agent - Staff Chair Crop, Soil and Environmental Sciences Department Johnson County University of Arkansas Division of Agriculture University of Arkansas Division of Agriculture Cooperative Extension Service Cooperative Extension Service Little Rock, Arkansas Clarksville, Arkansas Table of Contents Introduction . 4 Horsenettle . 17 Research Methods . 4 Horseweed . 18 Boom Sprayers . 5 Indigo, White Wild . 18 Boomless Sprayers . 5 Johnsongrass . 18 Guidance Systems . 5 Lyreleaf Sage . 19 Basal Bark and Stump Treatment . 6 Maypop . 19 Hack and Squirt . 6 Mayweed . 19 Individual Plant Treatment (IPT) . 7 Oaks . 20 Soil Spot Treatment . 7 Osage Orange . 20 Winter Weed Control in Bermudagrass . 7 Palmetto . 20 Clover Damage . 8 Perilla Mint . 21 Surfactant . 8 Persimmon . 21 Bermudagrass Sprigging and Weed Control . 8 Pigweed . 21 Bahiagrass . 9 Plaintain, Buckhorn . 22 Bitterweed . 9 Poison Hemlock . 22 Blackberry . 9 Poorjoe . 22 Brush, Mixed . 10 Prairie Tea . 23 Buckbrush . 10 Pricklypear . 23 Buffalo Bur . 10 Prickly Sida, Teaweed . 23 Buttercup . 11 Ragweed, Common . 24 Carpetweed . 11 Ragweed, Lanceleaf . 24 Chickweed, Common . 11 Red Cedar . 24 Chickweed, Sticky . 12 Red Sorrel . 25 Crabgrass . 12 Ryegrass . 25 Croton, Tropic . 12 Sandbur . 25 Croton, Woolly . 13 Sedges . 26 Dallisgrass . 13 Sericea Lespedeza . 26 Dewberry . 13 Spurge . 26 Dogfennel . 14 Sumac . 27 Downy Brome and Cheat . 14 Thistle, Bull . 27 Fescue, Tall . 14 Thistle, Milk . 27 Foxtail, Knotroot . 15 Thistle, Musk . 28 Greenbrier . 15 Thistle, Yellow . 28 Groundsel . 15 Texas Bullnettle .