Three Types of Acids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MDA Scientific SPM
MDA Scientific SPM Fast response monitor for the detection of a target gas SPM Advantages • Fast response monitor specific to target gas only • Gas sensitivity to ppb levels with physical evidence • Minimum maintenance and no dynamic calibration • Customized for harsh industrial environments • More than 50 gas calibrations available Applications • Outdoor locations • Corrosive areas • Remote sampling areas • Gas storage areas • Survey work • Perimeter/fencelines • Ventilation and exhaust systems Options • Z-purge system • Duty cycle • ChemKey™ • RS422 • Remote reset • Portable • Extended sample • Heater option (operate from -20°C to ±40°C) Technical Specification Specifications Detection Technique Chemcassette® Detection System Alarm Point Dual level alarms typically set at 1/2 TLV and TLV Response Time As fast as 10 seconds Alarm Indication Local audio/visual alarms; remote capability optional Signal Outputs SPDT concentration alarm relays; SPDT fault relay; 4-20 mA; digital display Relay Rating 120VAC@10amps; 240VAC@5amps; 48VDC@5amps Operating Temperature Range 32° to 104°F; 0° to 40°C (basic unit); heating/cooling optional Power Requirements 115/230 VAC 50/60 Hz, battery operation optional Enclosure NEMA 4X fiberglass (basic unit) Dimensions 12"(H) x 12"(W) x 7”(D) (30.5 x 30.5 x 17.8 cm) (basic unit) Weight Up to 25 pounds, depending on option installed Note: options may vary the specifications Detectable Gases Range Amines Ammonia (NH3) 2.6-75.0 ppm Ammonia (NH3)-II 2.6-75.0 ppm Dimethylamine (DMA) 0.1-6 ppm n-Butylamine (n-BA) 0.4-12 -

Vibrationally Excited Hydrogen Halides : a Bibliography On
VI NBS SPECIAL PUBLICATION 392 J U.S. DEPARTMENT OF COMMERCE / National Bureau of Standards National Bureau of Standards Bldg. Library, _ E-01 Admin. OCT 1 1981 191023 / oO Vibrationally Excited Hydrogen Halides: A Bibliography on Chemical Kinetics of Chemiexcitation and Energy Transfer Processes (1958 through 1973) QC 100 • 1X57 no. 2te c l !14 c '- — | NATIONAL BUREAU OF STANDARDS The National Bureau of Standards' was established by an act of Congress March 3, 1901. The Bureau's overall goal is to strengthen and advance the Nation's science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research and provides: (1) a basis for the Nation's physical measurement system, (2) scientific and technological services for industry and government, (3) a technical basis for equity in trade, and (4) technical services to promote public safety. The Bureau consists of the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Institute for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation's scientific community, industry, and commerce. The Institute consists of a Center for Radiation Research, an Office of Meas- urement Services and the following divisions: Applied Mathematics — Electricity — Mechanics — Heat — Optical Physics — Nuclear Sciences" — Applied Radiation 2 — Quantum Electronics 1 — Electromagnetics 3 — Time 3 1 1 and Frequency — Laboratory Astrophysics — Cryogenics . -

Naming Polyatomic Ions and Acids Oxyanions
Oxyanions Naming Polyatomic Ions and Oxyanions Acids Oxyanions- negative ions containing Oxyanions may contain the prefix oxygen. “hypo-”, less than, or “per-”, more than. These have the suffix “-ate” or “-ite” For example - “-ate” means it has more oxygen atoms ClO4 Perchlorate bonded, “-ite” has less - ClO3 Chlorate For example - ClO2 Chlorite 2- SO4 sulfate ClO- Hypochlorite 2- SO3 sulfite Acids Naming acids Naming Acids Certain compounds produce H+ ions in Does it contain oxygen? water, these are called acids. If it does not, it gets the prefix “hydro-” and If it does contain an oxyanion, then the suffix “-ic acid” replace the ending. You can recognize them because the neutral compound starts with “H”. HCl If the ending was “–ate”, add “-ic acid” Hydrochloric acid If the ending was “–ite”, add “-ous acid” For example HCl, H2SO4, and HNO3. HF Don’t confuse a polyatomic ion with a H2SO4 Sulfuric Acid Hydrofluoric acid neutral compound. H2SO3 Sulfurous Acid HCN HCO - is hydrogen carbonate, not an acid. 3 Hydrocyanic acid Examples Examples Nomenclature (naming) of Covalent compounds HNO3 HNO3 Nitric Acid HI HI Hydroiodic acid H3AsO4 H3AsO4 Arsenic Acid HClO2 HClO2 Chlorous Acid 1 Determining the type of bond Covalent bonding is very Covalent bonding is very First, determine if you have an ionic different from ionic naming compound or a covalent compound. similar to ionic naming A metal and a nonmetal will form an You always name the one that is least Ionic names ignored the subscript ionic bond. electronegative first (furthest from because there was only one possible Compounds with Polyatomic ions form fluorine) ratio of elements. -

Chemistry: the Molecular Nature of Matter and Change
REVISED CONFIRMING PAGES The Components of Matter 2.1 Elements, Compounds, and 2.5 The Atomic Theory Today 2.8 Formula, Name, and Mass of Mixtures: An Atomic Overview Structure of the Atom a Compound 2.2 The Observations That Led to Atomic Number, Mass Number, and Binary Ionic Compounds an Atomic View of Matter Atomic Symbol Compounds That Contain Polyatomic Mass Conservation Isotopes Ions Definite Composition Atomic Masses of the Elements Acid Names from Anion Names Multiple Proportions 2.6 Elements: A First Look at the Binary Covalent Compounds The Simplest Organic Compounds: Dalton’s Atomic Theory Periodic Table 2.3 Straight-Chain Alkanes Postulates of the Atomic Theory Organization of the Periodic Table Masses from a Chemical Formula How the Atomic Theory Explains Classifying the Elements Representing Molecules with a the Mass Laws Compounds: An Introduction 2.7 Formula and a Model to Bonding 2.4 The Observations That Led to Mixtures: Classification and the Nuclear Atom Model The Formation of Ionic Compounds 2.9 Separation Discovery of the Electron and The Formation of Covalent An Overview of the Components of Its Properties Compounds Matter Discovery of the Atomic Nucleus (a) (b) (right) ©Rudy Umans/Shutterstock IN THIS CHAPTER . We examine the properties and composition of matter on the macroscopic and atomic scales. By the end of this chapter, you should be able to • Relate the three types of matter—elements or elementary substances, compounds, and mixtures—to the simple chemical entities that they comprise—atoms, ions, and molecules; -

Hypochlorous Acid Handling
Hypochlorous Acid Handling 1 Identification of Petitioned Substance 2 Chemical Names: Hypochlorous acid, CAS Numbers: 7790-92-3 3 hypochloric(I) acid, chloranol, 4 hydroxidochlorine 10 Other Codes: European Community 11 Number-22757, IUPAC-Hypochlorous acid 5 Other Name: Hydrogen hypochlorite, 6 Chlorine hydroxide List other codes: PubChem CID 24341 7 Trade Names: Bleach, Sodium hypochlorite, InChI Key: QWPPOHNGKGFGJK- 8 Calcium hypochlorite, Sterilox, hypochlorite, UHFFFAOYSA-N 9 NVC-10 UNII: 712K4CDC10 12 Summary of Petitioned Use 13 A petition has been received from a stakeholder requesting that hypochlorous acid (also referred 14 to as electrolyzed water (EW)) be added to the list of synthetic substances allowed for use in 15 organic production and handling (7 CFR §§ 205.600-606). Specifically, the petition concerns the 16 formation of hypochlorous acid at the anode of an electrolysis apparatus designed for its 17 production from a brine solution. This active ingredient is aqueous hypochlorous acid which acts 18 as an oxidizing agent. The petitioner plans use hypochlorous acid as a sanitizer and antimicrobial 19 agent for the production and handling of organic products. The petition also requests to resolve a 20 difference in interpretation of allowed substances for chlorine materials on the National List of 21 Allowed and Prohibited Substances that contain the active ingredient hypochlorous acid (NOP- 22 PM 14-3 Electrolyzed water). 23 The NOP has issued NOP 5026 “Guidance, the use of Chlorine Materials in Organic Production 24 and Handling.” This guidance document clarifies the use of chlorine materials in organic 25 production and handling to align the National List with the November, 1995 NOSB 26 recommendation on chlorine materials which read: 27 “Allowed for disinfecting and sanitizing food contact surfaces. -

Acetate C2H3O2 Or CH3COO Or CH3CO2 Ammonium NH4
Name:_____________________ Memorization For AP Chem Polyatmic Ions AP Chemistry Polyatomic Ion list - - - Acetate C2H3O2 or CH 3COO or CH 3CO 2 + Ammonium NH 4 - Ammonium Carbonate NH 4CO 3 Cyanide CN - 2- Carbonate CO 3 2- Dichromate Cr 2O7 - Dihydrogen phosphate H2PO 4 - Hydrogen carbonate HCO 3 - Hydrogen sulfate HSO 4 - Hydronium H3O Hydroxide OH - 2- Monohydrogen Phosphate HPO 4 - Nitrate NO 3 - Nitrite NO 2 2- Oxalate C2O4 - Permanganate MnO 4 3- Phosphate PO 4 2- Sulfate SO 4 2- Sulfite SO 3 Hypochlorite ClO - - Chlorite ClO 2 - Chlorate ClO 3 - Perchlorate ClO 4 2- Chromate CrO 4 2- Silicate SiO 3 Thiocyanate SCN - 2- Thiosulfate S2O3 - Bromate BrO 3 2- Selenate SeO 4 - Bisulfite HSO 3 Name:_____________________ Memorization For AP Chem Rules for Naming an Acid When the name of the anion ends in –ide, the acid name begins with the prefix hydro-, the stem of the anion has the suffix –ic and it is followed by the word acid. -ide becomes hydro _____ic Acid Cl- is the Chloride ion so HCl = hydrochloric acid When the anion name ends in –ite, the acid name is the stem of the anion with the suffix –ous, followed by the word acid. -ite becomes ______ous Acid - ClO 2 is the Chlorite ion so HClO 2 = Chlorous acid. When the anion name ends in –ate, the acid name is the stem of the anion with the suffix –ic, followed by the word acid. -ate becomes ______ic Acid - ClO 3 is the Chlorate ion so HClO 3 = Chloric acid. Rules for Naming Ionic Compounds 1. -

Bimetallic Catalyst Catalyzed Carbonylation of Methanol to Acetic Acid
materials Article Study on Rh(I)/Ru(III) Bimetallic Catalyst Catalyzed Carbonylation of Methanol to Acetic Acid Shasha Zhang 1, Wenxin Ji 1,2,*, Ning Feng 2, Liping Lan 1, Yuanyuan Li 1,2 and Yulong Ma 1,2 1 College of Chemistry and Chemical Engineering, Ningxia University, Yinchuan 750021, China; [email protected] (S.Z.); [email protected] (L.L.); [email protected] (Y.L.); [email protected] (Y.M.) 2 State Key Laboratory of High-efficiency Utilization of Coal and Green Chemical Engineering, Ningxia University, Yinchuan 750021, China; [email protected] * Correspondence: [email protected]; Tel.: +86-135-1957-9989; Fax: +86-951-206-2323 Received: 13 July 2020; Accepted: 3 September 2020; Published: 11 September 2020 Abstract: In this study, a Rh(I)/Ru(III) catalyst with a bimetallic space structure was designed and synthesized. The interaction between the metals of the bimetallic catalyst and the structure of the bridged dimer can effectively reduce the steric hindrance effect and help speed up the reaction rate while ensuring the stability of the catalyst. X-ray photoelectron spectroscopy (XPS) results show that rhodium accepts electrons from chlorine, thereby increasing the electron-rich nature of rhodium and improving the catalytic activity. This promotes the nucleophilic reaction of the catalyst with methyl iodide and reduces the reaction energy barrier. The methanol carbonylation performance of the Rh/Ru catalyst was evaluated, and the results show that the conversion rate of methyl acetate and the yield of acetic acid are 96.0% under certain conditions. Furthermore, during the catalysis, no precipitate is formed and the amount of water is greatly reduced. -

Thursday 10 January 2019
Please check the examination details below before entering your candidate information Candidate surname Other names Pearson Edexcel Centre Number Candidate Number International Advanced Level Thursday 10 January 2019 Afternoon (Time: 1 hour 40 minutes) Paper Reference WCH04/01 Chemistry Advanced Unit 4: General Principles of Chemistry I – Rates, Equilibria and Further Organic Chemistry (including synoptic assessment) Candidates must have: Scientific calculator Total Marks Data Booklet Instructions • Use black ink or black ball-point pen. • Fill in the boxes at the top of this page with your name, centre number and candidate number. • Answer all questions. • Answer the questions in the spaces provided – there may be more space than you need. Information • The total mark for this paper is 90. • The marks for each question are shown in brackets – use this as a guide as to how much time to spend on each question. • Questions labelled with an asterisk (*) are ones where the quality of your written communication will be assessed – you should take particular care with your spelling, punctuation and grammar, as well as the clarity of expression, on these questions. • A Periodic Table is printed on the back cover of this paper. Advice • Read each question carefully before you start to answer it. • Show all your working in calculations and include units where appropriate. • Check your answers if you have time at the end. Turn over P54560A ©2019 Pearson Education Ltd. *P54560A0128* 2/1/1/1/1/1/ SECTION A Answer ALL the questions in this section. You should aim to spend no more than 20 minutes on THIS AREA WRITE IN DO NOT this section. -
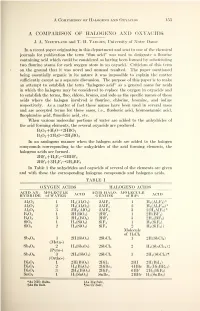
Proceedings of the Indiana Academy of Science
A Comparison of Halogeno and Oxyacids L53 A COMPARISON OF HALOGENO AND OXYACIDS J. A. Nieuwland and T. H. Vaughn, University of Notre Dame In a recent paper originating in this department and sent to one of the chemical journals for publication the term "fluo acid" was used to designate a flourine containing acid which could be considered as having been formed by substituting two flourine atoms for each oxygen atom in an oxyacid. Criticism of this term on the ground that it was novel and unusual resulted. The paper mentioned being essentially organic in its nature it was impossible to explain the matter sufficiently except as a separate discussion. The purpose of this paper is to make an attempt to establish the term "halogeno acid" as a general name for acids in which the halogens may be considered to replace the oxygen in oxyacids and to establish the terms, fluo, chloro, bromo, and iodo as the specific names of these acids where the halogen involved is flourine, chlorine, bromine, and iodine respectively. As a matter of fact these names have been used in several cases and are accepted terms for those cases, i.e., fluoboric acid, bromostannic acid, fluoplumbic acid, fluosilicic acid, etc. When various molecular portions of water are added to the anhydrides of the acid forming elements, the several oxyacids are produced. B 2 3 +H 2 0^2HB0 2 B 2 2 +3H 2 0->2H 3 B0 3 In an analogous manner when the halogen acids are added to the halogen compounds corresponding to the anhydrides of the acid forming elements, the halogeno acids are formed. -

The Summer Assignment Will Receive a GRADE on the First Day of Class – August 9
Bishop Moore AP Chemistry Summer Assignment June 2017 Future AP Chemistry Student, Welcome to AP Chemistry. In order to ensure the best start for everyone next fall, I have prepared a summer assignment that reviews basic chemistry concepts some of which you may have forgotten you learned. For those topics you need help with there are a multitude of tremendous chemistry resources available on the Internet. With access to hundreds of websites either in your home or at the local library, I am confident that you will have sufficient resources to prepare adequately for the fall semester. The reference text book as part of AP course is “Chemistry: The Central Science” by Brown LeMay 14th Edition for AP. Much of the material in this summer packet will be familiar to you. It will be important for everyone to come to class the first day prepared. While I review throughout the course, extensive remediation is not an option as we work towards our goal of being 100% prepared for the AP Exam in early May. There will be a test covering the basic concepts included in the summer packet during the first or second week of school. You may contact me by email: ([email protected]) this summer. I will do my best to answer your questions ASAP. I hope you are looking forward to an exciting year of chemistry. You are all certainly excellent students, and with plenty of motivation and hard work you should find AP Chemistry a successful and rewarding experience. Finally, I recommend that you spread out the summer assignment. -

Atoms, Molecules, Ions, and Inorganic Nomenclature
Atoms, Molecules, Ions, and Inorganic Nomenclature Brown, LeMay Ch 2 AP Chemistry Monta Vista High School 2.2: Evidence for the Atomic Theory 1. J.J. Thomson’s cathode ray tube: discovery of electrons and the e- charge-to-mass ratio ¶ In a vacuum chamber, flow of high voltage (emitted from cathode to anode) is deflected by magnetic & electrical fields (animation: http://highered.mcgraw-hill.com/olcweb/cgi/pluginpop.cgi?it=swf::100%::100%::/sites/dl/free/ 0072512644/117354/01_Cathode_Ray_Tube.swf::Cathode%20Ray%20Tube) 2 2. Robert Millikan’s oil drop: determines charge of e- (and thus the mass) ¶ “Atomized” drops of oil picked up small charges (integral numbers), and balanced oil drops in an electrical & gravitational field http://cwx.prenhall.com/petrucci/medialib/media_portfolio/text_images/004_MILLIKANOIL.MOV 3. Ernest Rutherford’s gold foil: discovery of nucleus as center of positive charge ¶ Alpha particles from radioactive source are deflected from positive gold atom nuclei http://www.mhhe.com/physsci/ chemistry/animations/ chang_2e/ rutherfords_experiment.swf 2.3: Structure of the Atom Figure 1: Subatomic particles (Table 2.1; 1 amu = 1.66054 x 10-24 g). Subatomic Charge Location Mass particle Proton, p+ +1.6 x 10-19 C nucleus 1.0073 amu Neutron, n None nucleus 1.0087 amu Electron, e- -1.6 x 10-19 C e- cloud 5.486 x 10-4 amu 5 Vocabulary n Atomic number: number of p+ (determines the element) n Mass number: sum of p+ and n (determines the isotope) n Isotopes: atoms of an element that differ in the number of neutrons n Isobars: atoms of different elements with same atomic mass but different atomic number. -

And Inter-Protein Couplings of Backbone Motions Underlie
www.nature.com/scientificreports OPEN Intra- and inter-protein couplings of backbone motions underlie protein thiol-disulfde exchange cascade Received: 26 July 2018 Wenbo Zhang1,3, Xiaogang Niu2,3, Jienv Ding1,3,5, Yunfei Hu2,3,6 & Changwen Jin1,2,3,4 Accepted: 6 October 2018 The thioredoxin (Trx)-coupled arsenate reductase (ArsC) is a family of enzymes that catalyzes the Published: xx xx xxxx reduction of arsenate to arsenite in the arsenic detoxifcation pathway. The catalytic cycle involves a series of relayed intramolecular and intermolecular thiol-disulfde exchange reactions. Structures at diferent reaction stages have been determined, suggesting signifcant conformational fuctuations along the reaction pathway. Herein, we use two state-of-the-art NMR methods, the chemical exchange saturation transfer (CEST) and the CPMG-based relaxation dispersion (CPMG RD) experiments, to probe the conformational dynamics of B. subtilis ArsC in all reaction stages, namely the enzymatic active reduced state, the intra-molecular C10–C82 disulfde-bonded intermediate state, the inactive oxidized state, and the inter-molecular disulfde-bonded protein complex with Trx. Our results reveal highly rugged energy landscapes in the active reduced state, and suggest global collective motions in both the C10–C82 disulfde-bonded intermediate and the mixed-disulfde Trx-ArsC complex. Protein thiol-disulfde exchange reactions play fundamental roles in living systems, represented by the thiore- doxin (Trx) and glutaredoxin (Grx) systems that maintain the cytoplasmic reducing environment, the protein DsbA that catalyzes the formation of protein disulfde bonds in bacterial periplasm, as well as the protein disulfde isomerase (PDI) proteins that facilitate correct disulfde bonding1–5.