Developmental, Ecological and Biological Drivers of Macroevolutionary Trajectories of Postcranial Morphological Diversification in Bats
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -

Special Publications Museum of Texas Tech University Number 63 18 September 2014
Special Publications Museum of Texas Tech University Number 63 18 September 2014 List of Recent Land Mammals of Mexico, 2014 José Ramírez-Pulido, Noé González-Ruiz, Alfred L. Gardner, and Joaquín Arroyo-Cabrales.0 Front cover: Image of the cover of Nova Plantarvm, Animalivm et Mineralivm Mexicanorvm Historia, by Francisci Hernández et al. (1651), which included the first list of the mammals found in Mexico. Cover image courtesy of the John Carter Brown Library at Brown University. SPECIAL PUBLICATIONS Museum of Texas Tech University Number 63 List of Recent Land Mammals of Mexico, 2014 JOSÉ RAMÍREZ-PULIDO, NOÉ GONZÁLEZ-RUIZ, ALFRED L. GARDNER, AND JOAQUÍN ARROYO-CABRALES Layout and Design: Lisa Bradley Cover Design: Image courtesy of the John Carter Brown Library at Brown University Production Editor: Lisa Bradley Copyright 2014, Museum of Texas Tech University This publication is available free of charge in PDF format from the website of the Natural Sciences Research Laboratory, Museum of Texas Tech University (nsrl.ttu.edu). The authors and the Museum of Texas Tech University hereby grant permission to interested parties to download or print this publication for personal or educational (not for profit) use. Re-publication of any part of this paper in other works is not permitted without prior written permission of the Museum of Texas Tech University. This book was set in Times New Roman and printed on acid-free paper that meets the guidelines for per- manence and durability of the Committee on Production Guidelines for Book Longevity of the Council on Library Resources. Printed: 18 September 2014 Library of Congress Cataloging-in-Publication Data Special Publications of the Museum of Texas Tech University, Number 63 Series Editor: Robert J. -

Timeline of the Evolutionary History of Life
Timeline of the evolutionary history of life This timeline of the evolutionary history of life represents the current scientific theory Life timeline Ice Ages outlining the major events during the 0 — Primates Quater nary Flowers ←Earliest apes development of life on planet Earth. In P Birds h Mammals – Plants Dinosaurs biology, evolution is any change across Karo o a n ← Andean Tetrapoda successive generations in the heritable -50 0 — e Arthropods Molluscs r ←Cambrian explosion characteristics of biological populations. o ← Cryoge nian Ediacara biota – z ← Evolutionary processes give rise to diversity o Earliest animals ←Earliest plants at every level of biological organization, i Multicellular -1000 — c from kingdoms to species, and individual life ←Sexual reproduction organisms and molecules, such as DNA and – P proteins. The similarities between all present r -1500 — o day organisms indicate the presence of a t – e common ancestor from which all known r Eukaryotes o species, living and extinct, have diverged -2000 — z o through the process of evolution. More than i Huron ian – c 99 percent of all species, amounting to over ←Oxygen crisis [1] five billion species, that ever lived on -2500 — ←Atmospheric oxygen Earth are estimated to be extinct.[2][3] Estimates on the number of Earth's current – Photosynthesis Pong ola species range from 10 million to 14 -3000 — A million,[4] of which about 1.2 million have r c been documented and over 86 percent have – h [5] e not yet been described. However, a May a -3500 — n ←Earliest oxygen 2016 -
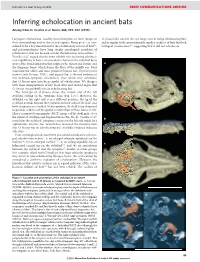
Inferring Echolocation in Ancient Bats Arising From: N
NATURE | Vol 466 | 19 August 2010 BRIEF COMMUNICATIONS ARISING Inferring echolocation in ancient bats Arising from: N. Veselka et al. Nature 463, 939–942 (2010) Laryngeal echolocation, used by most living bats to form images of O. finneyi falls outside the size range seen in living echolocating bats their surroundings and to detect and capture flying prey1,2, is con- and is similar to the proportionally smaller cochleae of bats that lack sidered to be a key innovation for the evolutionary success of bats2,3, laryngeal echolocation4,8, suggesting that it did not echolocate. and palaeontologists have long sought osteological correlates of echolocation that can be used to infer the behaviour of fossil bats4–7. Veselka et al.8 argued that the most reliable trait indicating echoloca- tion capabilities in bats is an articulation between the stylohyal bone (part of the hyoid apparatus that supports the throat and larynx) and a the tympanic bone, which forms the floor of the middle ear. They examined the oldest and most primitive known bat, Onychonycteris finneyi (early Eocene, USA4), and argued that it showed evidence of this stylohyal–tympanic articulation, from which they concluded that O. finneyi may have been capable of echolocation. We disagree with their interpretation of key fossil data and instead argue that O. finneyi was probably not an echolocating bat. The holotype of O. finneyi shows the cranial end of the left stylohyal resting on the tympanic bone (Fig. 1c–e). However, the stylohyal on the right side is in a different position, the tip of the stylohyal extends beyond the tympanic on both sides of the skull, and both tympanics are crushed. -

BIO 313 ANIMAL ECOLOGY Corrected
NATIONAL OPEN UNIVERSITY OF NIGERIA SCHOOL OF SCIENCE AND TECHNOLOGY COURSE CODE: BIO 314 COURSE TITLE: ANIMAL ECOLOGY 1 BIO 314: ANIMAL ECOLOGY Team Writers: Dr O.A. Olajuyigbe Department of Biology Adeyemi Colledge of Education, P.M.B. 520, Ondo, Ondo State Nigeria. Miss F.C. Olakolu Nigerian Institute for Oceanography and Marine Research, No 3 Wilmot Point Road, Bar-beach Bus-stop, Victoria Island, Lagos, Nigeria. Mrs H.O. Omogoriola Nigerian Institute for Oceanography and Marine Research, No 3 Wilmot Point Road, Bar-beach Bus-stop, Victoria Island, Lagos, Nigeria. EDITOR: Mrs Ajetomobi School of Agricultural Sciences Lagos State Polytechnic Ikorodu, Lagos 2 BIO 313 COURSE GUIDE Introduction Animal Ecology (313) is a first semester course. It is a two credit unit elective course which all students offering Bachelor of Science (BSc) in Biology can take. Animal ecology is an important area of study for scientists. It is the study of animals and how they related to each other as well as their environment. It can also be defined as the scientific study of interactions that determine the distribution and abundance of organisms. Since this is a course in animal ecology, we will focus on animals, which we will define fairly generally as organisms that can move around during some stages of their life and that must feed on other organisms or their products. There are various forms of animal ecology. This includes: • Behavioral ecology, the study of the behavior of the animals with relation to their environment and others • Population ecology, the study of the effects on the population of these animals • Marine ecology is the scientific study of marine-life habitat, populations, and interactions among organisms and the surrounding environment including their abiotic (non-living physical and chemical factors that affect the ability of organisms to survive and reproduce) and biotic factors (living things or the materials that directly or indirectly affect an organism in its environment). -

Carpe Noctem: the Importance of Bats As Bioindicators
Vol. 8: 93–115, 2009 ENDANGERED SPECIES RESEARCH Printed July 2009 doi: 10.3354/esr00182 Endang Species Res Published online April 8, 2009 Contribution to the Theme Section ‘Bats: status, threats and conservation successes’ OPENPEN REVIEW ACCESSCCESS Carpe noctem: the importance of bats as bioindicators Gareth Jones1,*, David S. Jacobs2, Thomas H. Kunz3, Michael R. Willig4, Paul A. Racey5 1School of Biological Sciences, University of Bristol, Woodland Road, Bristol BS8 1UG, UK 2Zoology Department, University of Cape Town, Rondesbosch 7701, Cape Town, South Africa 3Center for Ecology and Conservation Biology, Department of Biology, Boston University, Boston, Massachusetts 02215, USA 4Center for Environmental Sciences and Engineering and Department of Ecology and Evolutionary Biology, University of Connecticut, Storrs, Connecticut 06269-4210, USA 5School of Biological Sciences, University of Aberdeen, Tillydrone Avenue, Aberdeen AB24 2TN, UK ABSTRACT: The earth is now subject to climate change and habitat deterioration on unprecedented scales. Monitoring climate change and habitat loss alone is insufficient if we are to understand the effects of these factors on complex biological communities. It is therefore important to identify bioindicator taxa that show measurable responses to climate change and habitat loss and that reflect wider-scale impacts on the biota of interest. We argue that bats have enormous potential as bioindi- cators: they show taxonomic stability, trends in their populations can be monitored, short- and long- term effects on populations can be measured and they are distributed widely around the globe. Because insectivorous bats occupy high trophic levels, they are sensitive to accumulations of pesti- cides and other toxins, and changes in their abundance may reflect changes in populations of arthro- pod prey species. -

The Anatomy of the Hyoid Region of Molossus Molossus and Its Implication in Systematics
Illinois Wesleyan University Digital Commons @ IWU John Wesley Powell Student Research Conference 1991, 2nd Annual JWP Conference Apr 27th, 12:00 PM - 4:30 PM The Anatomy of the Hyoid Region of Molossus Molossus and its Implication in Systematics Natawadee Prasertphon Illinois Wesleyan University Thomas Griffiths,aculty F Advisor Illinois Wesleyan University Follow this and additional works at: https://digitalcommons.iwu.edu/jwprc Prasertphon, Natawadee and Griffiths, acultyF Advisor, Thomas, "The Anatomy of the Hyoid Region of Molossus Molossus and its Implication in Systematics" (1991). John Wesley Powell Student Research Conference. 14. https://digitalcommons.iwu.edu/jwprc/1991/posters/14 This is protected by copyright and/or related rights. It has been brought to you by Digital Commons @ IWU with permission from the rights-holder(s). You are free to use this material in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you need to obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This material has been accepted for inclusion by faculty at Illinois Wesleyan University. For more information, please contact [email protected]. ©Copyright is owned by the author of this document. THE ANATOMY OF THE HYOID REGION OF MOLOSSUS MOLOSSUS AND ITS IMPliCATION IN SYSTEMATICS Natawadee Prasertphon, Dept. of Biology, IWU, Thomas Griffiths* The hyoid musculature and hyoid apparatus of a bat, Molossus molossus (Chiroptera: Molossidae) is dissected and described. A comparison is made with the hyoid structures of bats of the genera Rhinopoma, Emballonura, Nycteris, Megaderma, Rhinoiophus, Pteronotus, Phyllostomus, and Ep tesicus, which were previously described by my sponsor Griffiths and associates. -

The Relationship Between Gene Network Structure and Expression Variation Among Individuals and Species
UC San Diego UC San Diego Previously Published Works Title The Relationship between Gene Network Structure and Expression Variation among Individuals and Species. Permalink https://escholarship.org/uc/item/77v6f7dc Journal PLoS genetics, 11(8) ISSN 1553-7390 Authors Sears, Karen E Maier, Jennifer A Rivas-Astroza, Marcelo et al. Publication Date 2015-08-28 DOI 10.1371/journal.pgen.1005398 Peer reviewed eScholarship.org Powered by the California Digital Library University of California RESEARCH ARTICLE The Relationship between Gene Network Structure and Expression Variation among Individuals and Species Karen E. Sears1,2*, Jennifer A. Maier1, Marcelo Rivas-Astroza3, Rachel Poe4, Sheng Zhong3, Kari Kosog1, Jonathan D. Marcot1, Richard R. Behringer5, Chris J. Cretekos6†, John J. Rasweiler, IV7, Zoi Rapti4 1 School of Integrative Biology, University of Illinois, Urbana, Illinois, United States of America, 2 Institute for Genomic Biology, University of Illinois, Urbana, Illinois, United States of America, 3 Department of Bioengineering, University of California, San Diego, La Jolla, California, United States of America, 4 Department of Mathematics, University of Illinois, Urbana, Illinois, United States of America, 5 Department of Genetics, University of Texas MD Anderson Cancer Center, Houston, Texas, United States of America, 6 Department of Biological Sciences, Idaho State University, Pocatello, Idaho, United States of America, 7 Department of Obstetrics and Gynecology, State University of New York Downstate Medical Center, Brooklyn, New York, United States of America † Deceased. * [email protected] OPEN ACCESS Citation: Sears KE, Maier JA, Rivas-Astroza M, Poe R, Zhong S, Kosog K, et al. (2015) The Relationship between Gene Network Structure and Expression Abstract Variation among Individuals and Species. -

Index of Handbook of the Mammals of the World. Vol. 9. Bats
Index of Handbook of the Mammals of the World. Vol. 9. Bats A agnella, Kerivoula 901 Anchieta’s Bat 814 aquilus, Glischropus 763 Aba Leaf-nosed Bat 247 aladdin, Pipistrellus pipistrellus 771 Anchieta’s Broad-faced Fruit Bat 94 aquilus, Platyrrhinus 567 Aba Roundleaf Bat 247 alascensis, Myotis lucifugus 927 Anchieta’s Pipistrelle 814 Arabian Barbastelle 861 abae, Hipposideros 247 alaschanicus, Hypsugo 810 anchietae, Plerotes 94 Arabian Horseshoe Bat 296 abae, Rhinolophus fumigatus 290 Alashanian Pipistrelle 810 ancricola, Myotis 957 Arabian Mouse-tailed Bat 164, 170, 176 abbotti, Myotis hasseltii 970 alba, Ectophylla 466, 480, 569 Andaman Horseshoe Bat 314 Arabian Pipistrelle 810 abditum, Megaderma spasma 191 albatus, Myopterus daubentonii 663 Andaman Intermediate Horseshoe Arabian Trident Bat 229 Abo Bat 725, 832 Alberico’s Broad-nosed Bat 565 Bat 321 Arabian Trident Leaf-nosed Bat 229 Abo Butterfly Bat 725, 832 albericoi, Platyrrhinus 565 andamanensis, Rhinolophus 321 arabica, Asellia 229 abramus, Pipistrellus 777 albescens, Myotis 940 Andean Fruit Bat 547 arabicus, Hypsugo 810 abrasus, Cynomops 604, 640 albicollis, Megaerops 64 Andersen’s Bare-backed Fruit Bat 109 arabicus, Rousettus aegyptiacus 87 Abruzzi’s Wrinkle-lipped Bat 645 albipinnis, Taphozous longimanus 353 Andersen’s Flying Fox 158 arabium, Rhinopoma cystops 176 Abyssinian Horseshoe Bat 290 albiventer, Nyctimene 36, 118 Andersen’s Fruit-eating Bat 578 Arafura Large-footed Bat 969 Acerodon albiventris, Noctilio 405, 411 Andersen’s Leaf-nosed Bat 254 Arata Yellow-shouldered Bat 543 Sulawesi 134 albofuscus, Scotoecus 762 Andersen’s Little Fruit-eating Bat 578 Arata-Thomas Yellow-shouldered Talaud 134 alboguttata, Glauconycteris 833 Andersen’s Naked-backed Fruit Bat 109 Bat 543 Acerodon 134 albus, Diclidurus 339, 367 Andersen’s Roundleaf Bat 254 aratathomasi, Sturnira 543 Acerodon mackloti (see A. -

Transcriptomic and Epigenomic Characterization of the Developing Bat Wing
ARTICLES OPEN Transcriptomic and epigenomic characterization of the developing bat wing Walter L Eckalbar1,2,9, Stephen A Schlebusch3,9, Mandy K Mason3, Zoe Gill3, Ash V Parker3, Betty M Booker1,2, Sierra Nishizaki1,2, Christiane Muswamba-Nday3, Elizabeth Terhune4,5, Kimberly A Nevonen4, Nadja Makki1,2, Tara Friedrich2,6, Julia E VanderMeer1,2, Katherine S Pollard2,6,7, Lucia Carbone4,8, Jeff D Wall2,7, Nicola Illing3 & Nadav Ahituv1,2 Bats are the only mammals capable of powered flight, but little is known about the genetic determinants that shape their wings. Here we generated a genome for Miniopterus natalensis and performed RNA-seq and ChIP-seq (H3K27ac and H3K27me3) analyses on its developing forelimb and hindlimb autopods at sequential embryonic stages to decipher the molecular events that underlie bat wing development. Over 7,000 genes and several long noncoding RNAs, including Tbx5-as1 and Hottip, were differentially expressed between forelimb and hindlimb, and across different stages. ChIP-seq analysis identified thousands of regions that are differentially modified in forelimb and hindlimb. Comparative genomics found 2,796 bat-accelerated regions within H3K27ac peaks, several of which cluster near limb-associated genes. Pathway analyses highlighted multiple ribosomal proteins and known limb patterning signaling pathways as differentially regulated and implicated increased forelimb mesenchymal condensation in differential growth. In combination, our work outlines multiple genetic components that likely contribute to bat wing formation, providing insights into this morphological innovation. The order Chiroptera, commonly known as bats, is the only group of To characterize the genetic differences that underlie divergence in mammals to have evolved the capability of flight. -

Appendix a Fauna Survey Effort and Results
APPENDIX A FAUNA SURVEY EFFORT AND RESULTS October 2016 Prepared by Anderson Environment and Planning The Fauna Survey Effort (FSE) for the Biobanking Assessment Report has been guided by the following: The predict threatened species from within the Biobanking Credit Calculator; The Threatened Species Survey and Assessment Guidelines for developments and activities (working draft), NSW Department of Environment and Conservation (2004); The NSW Threatened Species Profile Database; and Previous fauna survey results from the site. The following Ecosystem Credit species have been recorded on the site during past or current survey work: Eastern Bentwing-bat (Miniopterus schreibersii oceanensis); Eastern False Pipistrelle (Falsistrellus tasmaniensis); Eastern Freetail-bat (Mormopterus norfolkensis); Greater Broad-nosed Bat (Scoteanax rueppellii); Grey-headed Flying-fox (Pteropus poliocephalus); Little Bentwing-bat (Miniopterus australis); Little Lorikeet (Glossopsitta pusilla); Long-nosed Potoroo (Potorous tridactylus); Powerful Owl (Ninox strenua); Squirrel Glider (Petaurus norfolcensis); Varied Sittella (Daphoenositta chrysoptera); Yellow-bellied Glider (Petaurus australis); Yellow-bellied Sheathtail-bat (Saccolaimus flaviventris). The following Species Credit species have been recorded on the site or surrounds during past survey work: Koala (Phascolarctos cinereus); Wallum Froglet (Crinia tinnula). Prepared by Anderson Environment and Planning Contents 1.0 Introduction .............................................................................................................................................. -

Genetic and Molecular Control of Vertebrate Limb Development and Morphological Diversity
Genetic and Molecular Control of Vertebrate Limb Development and Morphological Diversity Lee Niswander HHMI/ U Colorado Health Sciences Center [email protected] Limb Development Chick Pro: experimental embryology, rapid functional analysis Con: relative lack of genetics Mouse Pro: genetics, genetic manipulation Con: in utero development Bat Pro: evo-devo studies, experimental embryology Con: relative lack of genetics relatively difficult to obtain Diversity of Diversity of Vertebrate Limbs Vertebrate Limbs A s= stylopod s Pr D z= zeugopod z a a= autopod P Modified from: Ridley M. Evolution, 2nd ed. A-P= Anterior-Posterior Blackwell Science 1996 Pr-D= Proximal-Distal P. Martin Int. J. Dev. Biol 1990 The thickened ectoderm at the distal rim of the limb bud is termed the apical ectodermal ridge. Laying down of the cartilage condensations E11.5 Martin, 1990 E12.5 Khokha et al. 2003 s z a Specification Elaboration of Patterning s z a 3 QuickTime™ and a 4 TIFF (LZW) decompressor 2 3 4 are needed to see this picture. 2 5 5 a z ssor ? ture. s pg pg S Z A Plzf/Gli3 dKO Barna et al. Fgf4/Fgf8 dKO Hoxa11/d11 dKO Sun et al. Nature (2002) Davis et al. Nature (1995) 1 Maria Barna AC D H Sin3A N-CoR/ SMRT POZ Zn+ Zn+ Zn+ Zn+ Zn+ Zn+ Zn+ Zn+ Zn+ PLZF (also known as ZFP 145) PLZF translocated to the Retinoic Acid Receptor Alpha (RARα)gene is associated with acute promyelocytic leukemia (APL) PLZF is a sequence specific DNA binding transcriptional repressor belonging to the POK (POZ and Kruppel) family of proteins that can remodel chromatin to a heterochromatic state.