Floral Herbivory Increases with Inflorescence Size and Local Plant
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lockerbie Wildlife Trust Eskrigg Reserve July 2016 News Bulletin
Lockerbie Wildlife Trust (www.lockerbie-wildlife-trust.co.uk) Eskrigg Reserve Scottish Charity No: July 2016 News Bulletin SC 005538 1. View of the pond from the Red Squirrel Hide on the 2nd of July and view of Eskrigg Centre and the Compost Toilet on the 6th of July. JR JR 2. Confirmed wildlife sightings at the Reserve in July. a. Birds CB Blackbird, Black East Indian Duck, Blue Tit, Bullfinch, Buzzard, Carrion Crow, Chaffinch, Chiffchaff, Coal Tit, Dunnock, Goldfinch, Great Spotted Woodpecker, Great Tit, Greenfinch, Grey Heron, Grey Wagtail, House Martin, House Sparrow, Jackdaw, Jay, Kestrel, Kingfisher, Little Grebe, Long-tailed Tit, Mallard, Moorhen, Mute Swan, Nuthatch, Pheasant, Raven, Robin, Siskin, Song Thrush, Sparrowhawk, Starling, Swallow, Swift, Treecreeper, Willow Warbler, Wood Pigeon, Wren. Kingfisher (Alcedo atthis) b. Mammals Bank Vole, Common Shrew, Fox, Mole, Rabbit, Red Squirrel, Roe Deer, Woodmouse. c. Reptiles and Amphibians Common Lizard, Frog, Newt, Toad. d. Invertebrates Black Slug; Buff-tailed, Tree and White-tailed Bumble Bees; JR Common Carder Bee; Green-veined White, Large Skipper, Large White, Meadow Brown, Ringlet and Small Tortoiseshell Butterflies; Crane-flies; Azure, Blue-tailed and Common Blue Damselflies; Common Darter and Four- spotted Chaser Dragonflies; Froghoppers, Grasshoppers; Dark Great Horse-fly; Foxglove Pug, Hoverflies; Eyed and 7-Spot Ladybird; Midges; Mosquitoes; Beautiful China- mark, Clouded Border, Common White Wave, July Foxglove Pug (Eupithecia plumbeolata) Highflyer, LargeYellow Underwing, -

Lepidoptera in Cheshire in 2002
Lepidoptera in Cheshire in 2002 A Report on the Micro-Moths, Butterflies and Macro-Moths of VC58 S.H. Hind, S. McWilliam, B.T. Shaw, S. Farrell and A. Wander Lancashire & Cheshire Entomological Society November 2003 1 1. Introduction Welcome to the 2002 report on lepidoptera in VC58 (Cheshire). This is the second report to appear in 2003 and follows on from the release of the 2001 version earlier this year. Hopefully we are now on course to return to an annual report, with the 2003 report planned for the middle of next year. Plans for the ‘Atlas of Lepidoptera in VC58’ continue apace. We had hoped to produce a further update to the Atlas but this report is already quite a large document. We will, therefore produce a supplementary report on the Pug Moths recorded in VC58 sometime in early 2004, hopefully in time to be sent out with the next newsletter. As usual, we have produced a combined report covering micro-moths, macro- moths and butterflies, rather than separate reports on all three groups. Doubtless observers will turn first to the group they are most interested in, but please take the time to read the other sections. Hopefully you will find something of interest. Many thanks to all recorders who have already submitted records for 2002. Without your efforts this report would not be possible. Please keep the records coming! This request also most definitely applies to recorders who have not sent in records for 2002 or even earlier. It is never too late to send in historic records as they will all be included within the above-mentioned Atlas when this is produced. -

Scottish Macro-Moth List, 2015
Notes on the Scottish Macro-moth List, 2015 This list aims to include every species of macro-moth reliably recorded in Scotland, with an assessment of its Scottish status, as guidance for observers contributing to the National Moth Recording Scheme (NMRS). It updates and amends the previous lists of 2009, 2011, 2012 & 2014. The requirement for inclusion on this checklist is a minimum of one record that is beyond reasonable doubt. Plausible but unproven species are relegated to an appendix, awaiting confirmation or further records. Unlikely species and known errors are omitted altogether, even if published records exist. Note that inclusion in the Scottish Invertebrate Records Index (SIRI) does not imply credibility. At one time or another, virtually every macro-moth on the British list has been reported from Scotland. Many of these claims are almost certainly misidentifications or other errors, including name confusion. However, because the County Moth Recorder (CMR) has the final say, dubious Scottish records for some unlikely species appear in the NMRS dataset. A modern complication involves the unwitting transportation of moths inside the traps of visiting lepidopterists. Then on the first night of their stay they record a species never seen before or afterwards by the local observers. Various such instances are known or suspected, including three for my own vice-county of Banffshire. Surprising species found in visitors’ traps the first time they are used here should always be regarded with caution. Clerical slips – the wrong scientific name scribbled in a notebook – have long caused confusion. An even greater modern problem involves errors when computerising the data. -

Latin Derivatives Dictionary
Dedication: 3/15/05 I dedicate this collection to my friends Orville and Evelyn Brynelson and my parents George and Marion Greenwald. I especially thank James Steckel, Barbara Zbikowski, Gustavo Betancourt, and Joshua Ellis, colleagues and computer experts extraordinaire, for their invaluable assistance. Kathy Hart, MUHS librarian, was most helpful in suggesting sources. I further thank Gaylan DuBose, Ed Long, Hugh Himwich, Susan Schearer, Gardy Warren, and Kaye Warren for their encouragement and advice. My former students and now Classics professors Daniel Curley and Anthony Hollingsworth also deserve mention for their advice, assistance, and friendship. My student Michael Kocorowski encouraged and provoked me into beginning this dictionary. Certamen players Michael Fleisch, James Ruel, Jeff Tudor, and Ryan Thom were inspirations. Sue Smith provided advice. James Radtke, James Beaudoin, Richard Hallberg, Sylvester Kreilein, and James Wilkinson assisted with words from modern foreign languages. Without the advice of these and many others this dictionary could not have been compiled. Lastly I thank all my colleagues and students at Marquette University High School who have made my teaching career a joy. Basic sources: American College Dictionary (ACD) American Heritage Dictionary of the English Language (AHD) Oxford Dictionary of English Etymology (ODEE) Oxford English Dictionary (OCD) Webster’s International Dictionary (eds. 2, 3) (W2, W3) Liddell and Scott (LS) Lewis and Short (LS) Oxford Latin Dictionary (OLD) Schaffer: Greek Derivative Dictionary, Latin Derivative Dictionary In addition many other sources were consulted; numerous etymology texts and readers were helpful. Zeno’s Word Frequency guide assisted in determining the relative importance of words. However, all judgments (and errors) are finally mine. -

Ecological Method Statement 22-01-2018
Brindle & Green Ecological Consultants Ltd ECOLOGISTS FOR BUSINESS www.brindlegreen.co.uk TEL: 0800 222 9105 Planning Services Peak District National Park Authority c/o Mr K Clayton Acumen Designers and Architects Ltd Headrow House Old Leeds Road Huddersfield HD1 1SG 10th January 2018 FAO: Mr John Scott RE: BG17.320 (Adaption of stables to form holiday accommodation NP/HPK/1015/0926, Park Hall Manor, Little Hayfield, Glossop, SK22 2NN) – Ecological Method Statement Dear John, I write to provide you with an ecological method statement pertaining to bats following emergence surveys undertaken at Park Hall Manor, Little Hayfield in order to discharge condition 6, associated with planning application NP/HPK/1015/0926, which stated: “No development shall take place until full details of an Ecological Method Statement have been submitted to and approved in writing by the Planning Authority. These details shall include: • Details of a working method statement to show how the proposed works will be undertaken in a manner that minimises the potential disturbance to bats and safeguards the value of the roost. • Details of bat friendly lighting, demonstrating avoidance of light spill onto roosts and provision of dark corridors for commuting bats. • Details of wildlife friendly planting and other ecological enhancements as deemed appropriate.” Summary Brindle & Green were commissioned by Leon Van Tonder to undertake bat emergence/re-entry surveys on a single storey stable block known as ‘The Stables’ at Park Hall Manor, Little Hayfield, prior to the buildings conversion into a residential dwelling. The bat surveys were undertaken during July, August and September 2015. The purpose of the bat surveys was to determine whether bats were using ‘The Stables’ building as a roosting site and to assess the level and type of bat activity on site. -
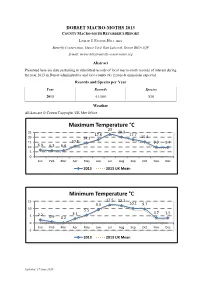
Dorset Macro-Moths 2013 County Macro-Moth Recorder's Report
DORSET MACRO-MOTHS 2013 COUNTY MACRO-MOTH RECORDER'S REPORT LESLIE J. EVANS-HILL FRES Butterfly Conservation, Manor Yard, East Lulworth, Dorset BH20 5QP E-mail: [email protected] Abstract Presented here are data pertaining to submitted records of local macro-moth records of interest during the year 2013 in Dorset administrative and vice-county (9). Errors & omissions expected. Records and Species per Year Year Records Species 2013 61,580 538 Weather All data are © Crown Copyright. UK Met Office. Maximum Temperature °C 23 25 20.3 17.6 17.7 20 14.1 15.4 15 10.8 9.9 9.7 10 6.9 6.3 6.4 5 0 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec 2013 2013 UK Mean Minimum Temperature °C 15 12.5 12.1 9.3 10.1 9.7 10 5.5 3.1 3.7 3.5 5 2.2 0.9 0.2 0 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec -5 2013 2013 UK Mean Updated: 17 June 2020 Sunshine Hours 400 297.7 300 206.8 195 161.1 177.3 200 107.5 68.8 94.6 88.6 68.8 100 44.9 44.5 0 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec 2013 2013 UK Mean Rainfall mm 250 212.3 217.2 200 144.7 150 104.3 91 92 86.9 72.9 100 61 46.8 49.4 45.1 50 0 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec 2013 2013 UK Mean Days of Rain >=1mm 21 25 18.3 20.2 20 14.7 11.6 10.7 11.8 15 8.6 10.3 8.8 10.6 10 6.5 5 0 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec 2013 2013 UK Mean Updated: 17 June 2020 Days of Air Frost 25 20 16.8 15 11 10.4 7.5 10 4.7 2.8 5 0.5 0 0 0 0 0 0 -5 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec 2013 2013 UK Mean New to Dorset One species is confirmed new to Dorset during 2013: • Chiasmia aestimaria (Hübner, [1809]) TAMARISK PEACOCK from Portland (SY6868); 1 Adult on 26 Aug 2013 by M Cade. -

Loch Leven Species Count
Loch Leven Species Count Preferred name Common name Coccinella septempunctata 7-spot Ladybird Alnus glutinosa Alder Persicaria amphibia Amphibious Bistort Poa annua Annual Meadow-grass Cerapteryx graminis Antler Moth Fraxinus excelsior Ash Populus tremula Aspen Scorzoneroides autumnalis Autumn Hawkbit Coenagrion puella Azure Damselfly Nemotelus uliginosus Barred Snout Gandaritis pyraliata Barred Straw Nymphula nitidulata Beautiful China-mark Autographa pulchrina Beautiful Golden Y Fagus sylvatica Beech Erica cinerea Bell Heather Vaccinium myrtillus Bilberry Bombus monticola Bilberry Bumblebee Yponomeuta evonymella Bird-cherry Ermine Chroicocephalus ridibundus Black-headed Gull Black East Indian Duck Black East Indian Duck Chrysopilus cristatus Black Snipefly Turdus merula Blackbird Sylvia atricapilla Blackcap Prunus spinosa Blackthorn Ischnura elegans Blue-tailed Damselfly Cyanistes caeruleus Blue Tit Pteridium aquilinum Bracken Lacanobia oleracea Bright-line Brown-eye Gonepteryx rhamni rhamni Brimstone Rumex obtusifolius Broad-leaved Dock Epilobium montanum Broad-leaved Willowherb Dryopteris dilatata Broad Buckler-fern Chloromyia formosa Broad Centurion Bombus humilis Brown-banded Carder Bee Rattus norvegicus Brown Rat Bombus terrestris Buff-tailed Bumblebee Anchusa arvensis Bugloss Diachrysia chrysitis Burnished Brass Buteo buteo Buzzard Corvus corone Carrion Crow Corvus corone subsp. corone Carrion Crow Hypochaeris radicata Cat's-ear Fringilla coelebs Chaffinch Tyria jacobaeae Cinnabar Galium aparine Cleavers Apamea crenata Clouded-bordered -
Common Name Species Name
Species Recorded at Wentworth Garden Centre By Members of South Yorkshire British Naturalists Association January 2014 to December 2017 Species Common Name Species Name Birds Birds (Drop-ins) Black Headed Gull Chroicocephalus ridibundus Birds (Regular) Blackbird Turdus merula Birds (Drop-ins) Blackcap Sylvia atricapilla Birds (Regular) Blue Tit Parus caerulrus Birds (Drop-ins) Brambling Fringilla montifringilla Birds (Drop-ins) Bullfinch Pyrrhula pyrrhula Birds (Occassional) Buzzard Buteo buteo Birds (Drop-ins) Canada Goose Branta canadensis Birds (Occassional) Carrion Crow Corvus corone Birds (Occassional) Chaffinch Fringilla coelebs Birds (Drop-ins) Chiffchaff Phylloscopus collybita Birds (Occassional) Coal Tit Parus ater Birds (Drop-ins) Collared Dove Streptopelia decaocto Birds (Drop-ins) Cormorant Phalacrocorax carbo Birds (Occassional) Dunnock Prunella modularis Birds (Drop-ins) Fieldfare Turdus pilaris Birds (Drop-ins) Garden Warbler Sylvia borin Birds (Occassional) Goldcrest Regulus regulus Birds (Drop-ins) Golden Plover Pluvialis apricaria Birds (Regular) Goldfinch Carduelis carduelis Birds (Drop-ins) Goosander Megus merganser Birds (Drop-ins) Great Black Backed Gull Larus marinus Birds (Drop-ins) Great Spotted Woodpecker Dendrocopos major Birds (Occassional) Great Tit Parus major Birds (Drop-ins) Green Woodpecker Picus viridis Birds (Occassional) Greenfinch Carduelis chloris Birds (Drop-ins) Grey Heron Ardea cinerea Birds (Drop-ins) Grey Wagtail Motacilla cinerea Birds (Drop-ins) Greylag goose Anser anser Birds (Drop-ins) Herring -
Ireland Red List No. 9: Macro-Moths (Lepidoptera)
Ireland Red List No. 9 Macro-moths (Lepidoptera) Ireland Red List No. 9 Macro-moths (Lepidoptera) D. Allen1, M. O’Donnell2, B. Nelson3, A. Tyner4, K.G.M. Bond5, T. Bryant6, A. Crory7, C. Mellon1, J. O’Boyle8, E. O’Donnell9, T. Rolston10, R. Sheppard11, P. Strickland12, U. Fitzpatrick13, E. Regan14. 1Allen & Mellon Environmental Ltd, 21A Windor Avenue, Belfast, BT9 6EE 2Joffre Rose, Clone, Castletown, Gorey, Co. Wexford 3National Parks & Wildlife Service, Department of the Arts, Heritage and the Gaeltacht, Ely Place, Dublin D02 TW98 4Honeyoak, Cronykeery, Ashford, Co. Wicklow 5Zoology, Ecology and Plant Science, Distillery Fields, North Mall, University College Cork 6Knocknarea, Priest’s Road, Tramore, Co. Waterford 7113 Dundrum Road, Newcastle, Co. Down, BT33 0LN 8Natural Environment Division, Northern Ireland Environment Agency, Department of Agriculture, Environment and Rural Affairs, Klondyke Building, Cromac Avenue, Belfast, BT7 2JA 95 Forgehill Rise, Stamullen, Co. Meath 1042 Beechdene Gardens, Lisburn, Co. Antrim, BT28 3JH 11Carnowen, Raphoe, Co. Donegal 1222 Newtown Court, Maynooth, Co. Kildare 13National Biodiversity Data Centre, WIT west campus, Carriganore, Waterford 14The Biodiversity Consultancy, 3E King’s Parade, Cambridge, CB2 1SJ Citation: Allen, D., O’Donnell, M., Nelson, B., Tyner, A., Bond, K.G.M., Bryant, T., Crory, A., Mellon, C., O’Boyle, J., O’Donnell, E., Rolston, T., Sheppard, R., Strickland, P., Fitzpatrick, U., & Regan, E. (2016) Ireland Red List No. 9: Macro-moths (Lepidoptera). National Parks and Wildlife Service, Department of Arts, Heritage and the Gaeltacht, Dublin, Ireland. Cover photos: Bottom left to top right: White Prominent Leucodonta bicoloria—photo: Brian Nelson; Burren Green Calamia tridens—photo: Brian Nelson; Figure of Eight Diloba caeruleocephala caterpillar—photo: Geoff Campbell; Thrift Clearwing Pyropteron muscaeformis— photo: Eamonn O’Donnell; Yellow Shell Camptogramma bilineata—photo: Geoff Campbell. -
Haslemere Bioblitz 2019 Report
Haslemere Natural History Society Haslemere BioBlitz 2019 Report Introduction The first Haslemere BioBlitz took place in the period 28-30th June 2019, targeting the area within 1km of Haslemere Town Hall, and was organised by Haslemere Natural History Society (HNHS). There was particular focus on the grounds of Haslemere Educational Museum and the National Trust’s Swan Barn Farm, with support from those two organisations. The intention was to involve invited experts, HNHS members and the general public in the search for as many species of macroscopic organisms as possible. The purposes of the event were both a) scientific – to establish a base-line of data and potentially to discover species of conservation significance; and b) educational – to involve and enthuse people in the study of our local biodiversity. Methods Schedule The event compiled records of species sighted in the period 10.00am Friday 28th June to 10.00am Sunday 30th June. Records from HNHS members, invited experts and the general public were welcomed for the whole of that period, but there were thematic events on these days for HNHS members and/or the public that also generated data. On 28th June, the weather was sunny but breezy. 29th June was very warm, and was followed by a warm, humid night. Field Techniques In addition to general observation and searches, the following focussed techniques were used: Camera trap: an infra-red “trail camera” was used in the Museum grounds and in woodland close to Swan Barn Farm. Bat detectors: heterodyne detectors were used during a bat walk at Swan Barn Farm; an ultra- sound recording and automatic diagnosis system was used overnight at a Haslemere house as well as during the bat walk. -
Lancashire, Manchester and Merseyside Butterfly and Moth Recording Report
BUTTERFLY CONSERVATION LANCASHIRE BRANCH DEDICATED TO SAVING WILD BUTTERFLIES, MOTHS AND THEIR HABITATS Lancashire, Manchester and Merseyside Butterfly and Moth Recording Report 2014 Butterfly Conservation President Sir David Attenborough Registerd Office Manor Yard, East Lulworth, Wareham, Dorset BH20 5QP Head Office Manor Yard, East Lulworth, Wareham, Dorset BH205QP Registered in England 2206468 Tel 0870 7744309 Fax 0870 7706150 Registered Charity No 254397 Email: [email protected] Sivell Butterfly Conservation Registered in England 2206468 Pete Marsh Registered Charity 254937 President Sir David Attenborough John Girdley Head Office Manor Yard, East Lulworth, Wareham, Dorset BH20 5QP 01929 400209 Butterfly Recording Laura Sivell County Butterfly Recorder Please continue to send your butterfly records (remember, every little helps)to: Lancashire and Merseyside Laura Sivell, email [email protected]. Or by post to 22 Beaumont Place, Lancaster LA1 2EY. Phone 01524 69248. Please note that for records to be included in the annual report, the deadline is the end of February. Late records will still be used for the database, but once the report is written, I’m not going to update or rewrite on the basis of late records. The report is also going to have to be written earlier in the year, in February, as I’m full on with work in March/April and I just can’t do it! East Lancs John Plackett ([email protected]) from Nelson Nats has taken over the East Lancashire Butterfly Report, so please all send your records to him as well as the Lancashire recorder. Greater Manchester These records should only go to Peter Hardy, 81 Winstanley Road, Sale, M33 2AT, email [email protected] - not to Laura Sivell. -

Moths and Butterflies of Keele.Pdf
THE BUTTERFLIES and MOTHS of KEELE UNIVERSITY David W. Emley Keele University Library Occasional Publication No. 18 1982 2 ACKNOWLEDGMENTS I would like to thank Mr. Sherratt of the Department of Biological Sciences for allowing me to use the Departments’ moth-trap, without which this booklet would not have been possible. I thank Dr. M. Majerus and Dr. J. Harrison also of that department for giving me records and helping me to run the trap. I thank Mr. G. Barber of the Geography Department for drawing the map and Dr. B.K. Holdsworth and Mr. G. Lees for reading the script. Finally I thank Miss P.J. Haselock for her excellent drawings, for reading and commenting on the text and for helping me to operate the moth trap. Further Reading South, R. The Moths of the British Isles. Warne Ford, E. B. Butterflies. Collins. Ford, E. B. Moths. Collins. Ford, R.L.E. Observers Book of Larger Moths. Warne. Howarth, T.G. Colour Identification Guide to British Butterflies. Warne. Novak, I. A Field Guide in Colour to Butterflies and Moths. Octopus. Newman, L.H. Looking at Butterflies. Collins. Stokoe, W.J. Observers Book of Butterflies. Warne. Higgins, R. & Riley, N.D. A field Guide to the Butterflies of Britain and Europe. Collins. Heath, R. Ed. Moths and Butterflies of Britain and Ireland. Curwen. 3 The Butterflies and Moths of Keele University Introduction Keele grounds are covered with a wide range of trees, shrubs and flowering plants which in turn support a rich insect and bird fauna. The system of footpaths that criss-crosses the area enables us to study this wealth of wildlife with ease.