Structure, Controllable Synthesis and Applications in Catalysis, Energy and Sensing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

I Want to Be More Hong Kong Than a Hongkonger”: Language Ideologies and the Portrayal of Mainland Chinese in Hong Kong Film During the Transition
Volume 6 Issue 1 2020 “I Want to be More Hong Kong Than a Hongkonger”: Language Ideologies and the Portrayal of Mainland Chinese in Hong Kong Film During the Transition Charlene Peishan Chan [email protected] ISSN: 2057-1720 doi: 10.2218/ls.v6i1.2020.4398 This paper is available at: http://journals.ed.ac.uk/lifespansstyles Hosted by The University of Edinburgh Journal Hosting Service: http://journals.ed.ac.uk/ “I Want to be More Hong Kong Than a Hongkonger”: Language Ideologies and the Portrayal of Mainland Chinese in Hong Kong Film During the Transition Charlene Peishan Chan The years leading up to the political handover of Hong Kong to Mainland China surfaced issues regarding national identification and intergroup relations. These issues manifested in Hong Kong films of the time in the form of film characters’ language ideologies. An analysis of six films reveals three themes: (1) the assumption of mutual intelligibility between Cantonese and Putonghua, (2) the importance of English towards one’s Hong Kong identity, and (3) the expectation that Mainland immigrants use Cantonese as their primary language of communication in Hong Kong. The recurrence of these findings indicates their prevalence amongst native Hongkongers, even in a post-handover context. 1 Introduction The handover of Hong Kong to the People’s Republic of China (PRC) in 1997 marked the end of 155 years of British colonial rule. Within this socio-political landscape came questions of identification and intergroup relations, both amongst native Hongkongers and Mainland Chinese (Tong et al. 1999, Brewer 1999). These manifest in the attitudes and ideologies that native Hongkongers have towards the three most widely used languages in Hong Kong: Cantonese, English, and Putonghua (a standard variety of Mandarin promoted in Mainland China by the Government). -

Tissue-Restricted Genome Editing in Vivo Specified by Microrna-Repressible Anti-CRISPR Proteins
Downloaded from rnajournal.cshlp.org on September 29, 2021 - Published by Cold Spring Harbor Laboratory Press REPORT Tissue-restricted genome editing in vivo specified by microRNA-repressible anti-CRISPR proteins JOOYOUNG LEE,1 HAIWEI MOU,1,4 RAED IBRAHEIM,1 SHUN-QING LIANG,1 PENGPENG LIU,2 WEN XUE,1,3 and ERIK J. SONTHEIMER1,3 1RNA Therapeutics Institute, University of Massachusetts Medical School, Worcester, Massachusetts 01605, USA 2Program in Molecular, Cell and Cancer Biology, University of Massachusetts Medical School, Worcester, Massachusetts 01605, USA 3Program in Molecular Medicine, University of Massachusetts Medical School, Worcester, Massachusetts 01605, USA ABSTRACT CRISPR-Cas systems are bacterial adaptive immune pathways that have revolutionized biotechnology and biomedical ap- plications. Despite the potential for human therapeutic development, there are many hurdles that must be overcome be- fore its use in clinical settings. Some clinical safety concerns arise from editing activity in unintended cell types or tissues upon in vivo delivery (e.g., by adeno-associated virus (AAV) vectors). Although tissue-specific promoters and serotypes with tissue tropisms can be used, suitably compact promoters are not always available for desired cell types, and AAV tis- sue tropism specificities are not absolute. To reinforce tissue-specific editing, we exploited anti-CRISPR proteins (Acrs) that have evolved as natural countermeasures against CRISPR immunity. To inhibit Cas9 in all ancillary tissues without compro- mising editing in the target tissue, we established a flexible platform in which an Acr transgene is repressed by endoge- nous, tissue-specific microRNAs (miRNAs). We demonstrate that miRNAs regulate the expression of an Acr transgene bearing miRNA-binding sites in its 3′′′′′-UTR and control subsequent genome editing outcomes in a cell-type specific manner. -

Origin Narratives: Reading and Reverence in Late-Ming China
Origin Narratives: Reading and Reverence in Late-Ming China Noga Ganany Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2018 © 2018 Noga Ganany All rights reserved ABSTRACT Origin Narratives: Reading and Reverence in Late Ming China Noga Ganany In this dissertation, I examine a genre of commercially-published, illustrated hagiographical books. Recounting the life stories of some of China’s most beloved cultural icons, from Confucius to Guanyin, I term these hagiographical books “origin narratives” (chushen zhuan 出身傳). Weaving a plethora of legends and ritual traditions into the new “vernacular” xiaoshuo format, origin narratives offered comprehensive portrayals of gods, sages, and immortals in narrative form, and were marketed to a general, lay readership. Their narratives were often accompanied by additional materials (or “paratexts”), such as worship manuals, advertisements for temples, and messages from the gods themselves, that reveal the intimate connection of these books to contemporaneous cultic reverence of their protagonists. The content and composition of origin narratives reflect the extensive range of possibilities of late-Ming xiaoshuo narrative writing, challenging our understanding of reading. I argue that origin narratives functioned as entertaining and informative encyclopedic sourcebooks that consolidated all knowledge about their protagonists, from their hagiographies to their ritual traditions. Origin narratives also alert us to the hagiographical substrate in late-imperial literature and religious practice, wherein widely-revered figures played multiple roles in the culture. The reverence of these cultural icons was constructed through the relationship between what I call the Three Ps: their personas (and life stories), the practices surrounding their lore, and the places associated with them (or “sacred geographies”). -

Representing Talented Women in Eighteenth-Century Chinese Painting: Thirteen Female Disciples Seeking Instruction at the Lake Pavilion
REPRESENTING TALENTED WOMEN IN EIGHTEENTH-CENTURY CHINESE PAINTING: THIRTEEN FEMALE DISCIPLES SEEKING INSTRUCTION AT THE LAKE PAVILION By Copyright 2016 Janet C. Chen Submitted to the graduate degree program in Art History and the Graduate Faculty of the University of Kansas in partial fulfillment of the requirements for the degree of Doctor of Philosophy. ________________________________ Chairperson Marsha Haufler ________________________________ Amy McNair ________________________________ Sherry Fowler ________________________________ Jungsil Jenny Lee ________________________________ Keith McMahon Date Defended: May 13, 2016 The Dissertation Committee for Janet C. Chen certifies that this is the approved version of the following dissertation: REPRESENTING TALENTED WOMEN IN EIGHTEENTH-CENTURY CHINESE PAINTING: THIRTEEN FEMALE DISCIPLES SEEKING INSTRUCTION AT THE LAKE PAVILION ________________________________ Chairperson Marsha Haufler Date approved: May 13, 2016 ii Abstract As the first comprehensive art-historical study of the Qing poet Yuan Mei (1716–97) and the female intellectuals in his circle, this dissertation examines the depictions of these women in an eighteenth-century handscroll, Thirteen Female Disciples Seeking Instructions at the Lake Pavilion, related paintings, and the accompanying inscriptions. Created when an increasing number of women turned to the scholarly arts, in particular painting and poetry, these paintings documented the more receptive attitude of literati toward talented women and their support in the social and artistic lives of female intellectuals. These pictures show the women cultivating themselves through literati activities and poetic meditation in nature or gardens, common tropes in portraits of male scholars. The predominantly male patrons, painters, and colophon authors all took part in the formation of the women’s public identities as poets and artists; the first two determined the visual representations, and the third, through writings, confirmed and elaborated on the designated identities. -

Names of Chinese People in Singapore
101 Lodz Papers in Pragmatics 7.1 (2011): 101-133 DOI: 10.2478/v10016-011-0005-6 Lee Cher Leng Department of Chinese Studies, National University of Singapore ETHNOGRAPHY OF SINGAPORE CHINESE NAMES: RACE, RELIGION, AND REPRESENTATION Abstract Singapore Chinese is part of the Chinese Diaspora.This research shows how Singapore Chinese names reflect the Chinese naming tradition of surnames and generation names, as well as Straits Chinese influence. The names also reflect the beliefs and religion of Singapore Chinese. More significantly, a change of identity and representation is reflected in the names of earlier settlers and Singapore Chinese today. This paper aims to show the general naming traditions of Chinese in Singapore as well as a change in ideology and trends due to globalization. Keywords Singapore, Chinese, names, identity, beliefs, globalization. 1. Introduction When parents choose a name for a child, the name necessarily reflects their thoughts and aspirations with regards to the child. These thoughts and aspirations are shaped by the historical, social, cultural or spiritual setting of the time and place they are living in whether or not they are aware of them. Thus, the study of names is an important window through which one could view how these parents prefer their children to be perceived by society at large, according to the identities, roles, values, hierarchies or expectations constructed within a social space. Goodenough explains this culturally driven context of names and naming practices: Department of Chinese Studies, National University of Singapore The Shaw Foundation Building, Block AS7, Level 5 5 Arts Link, Singapore 117570 e-mail: [email protected] 102 Lee Cher Leng Ethnography of Singapore Chinese Names: Race, Religion, and Representation Different naming and address customs necessarily select different things about the self for communication and consequent emphasis. -

“A Place of Reflection: Po-Wen Liu,” Ceramics Monthly
A Place of Reflection: Po-Wen Liu By: Elizabeth Perrill Elizabeth Perrill, “A Place of Reflection: Po-Wen Liu,” Ceramics Monthly. v62 n8 (Oct 2014) 34-37. Originally published in the October 2014 issue of Ceramics Monthly, pages (34–37). http://www.ceramicsmonthly.org. Copyright, The American Ceramic Society. Reprinted with permission. Abstract: The article discusses the work of ceramic artist Po-Wen Liu. Topics include the influence of North Carolina's Blue Ridge Piedmont region on Liu's work, Buddhist influence on Liu's works, and the use of the lotus as a motif in his pottery. He use of glazing techniques including celadon glazing, monochromatic glazing, and alkaline-glaze formulas is noted. Keywords: Po-Wen Liu | ceramics | pottery Article: ***Note: Full text of article below Po-Wen Liu by Dr. Elizabeth Perrill Uprooting one’s self and moving to a new physical location is often a moment for transformation and reflection. This truism can be even more poignant for ceramicists; those who are invested in the history of local ceramic traditions or inspired by specificities of land and architecture are par ticularly vulnerable to the travails of displace ment. Amidst the exigencies of professional or academic life, the question, “Where is your place?” can become a source of unsettling challenges. In the past decade, Po-Wen Liu, has been no stranger to moving both his home and his studio. Relocating from his native Taiwan, where he earned a degree in ceramic engineering at the competitive National Lian-Ho University, Liu first acquired a BFA in ceramics from the School of American Craft in Rochester, New York in 2000. -

Julianne Kimmel,1 BSN Student & Jia-Wen Guo,1 Phd, RN
TEXT ANALYSIS OF SUICIDAL THOUGHTS AND BEHAVIORS IN ADOLESCENTS AND YOUNG ADULTS Julianne Kimmel,1 BSN student & Jia-Wen Guo,1 PhD, RN 1 University of Utah College of Nursing Introduction Methods Results Conclusions In recent years, it has become This study involved a secondary data analysis of an existing Of the total words taken from the survey, 200 frequently used words The key findings of the study are that frequently evident that the number of patients dataset. AYAs were asked to provide brief descriptions of how that presented at least three times, 115 (57%) were directly describing used words directly related to suicidal thoughts or in the adolescent and young adult they and their peers expressed their experiences of self-harm, suicide-related thoughts or behaviors. Of those words, the top two behaviors and the specific terms (e.g., slangs) used (AYA) age category (11-25 years) suicidal thinking and attempts. most frequently used words were cut (126 uses) and kill (110 uses). by the AYA generation are useful for the early experiencing suicide ideation (SI), A quantitative text analysis was conducted to discover Several teen slang words or social media terms were found (Table 1). detection of suicide ideation and intention from suicide attempts (SA) and self-harm frequently used words and themes that emerged from the With the strong generational ties that most of these words have, it is the social media posts. has grown exponentially and it a suicide-related texts. Word frequency and word co-occurrence highly unlikely that the general public would not view these words as The slang words offer a look into the vernacular grave threat to this population network from the text data were examined by using KH Coder, alarming and maybe even confusing. -
C442 A19-Syllabus Schedule
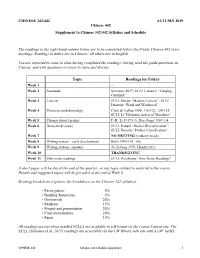
CHINESE 342/442 AUTUMN 2019 Chinese 442 Supplement to Chinese 342/442 Syllabus and Schedule The readings in the right-hand column below are to be completed before the Friday Chinese 442 class meetings. Readings in italics are in Chinese; all others are in English. You are expected to come to class having completed the readings; having read the guide questions on Canvas; and with questions or issues to raise and discuss. Topic Readings for Friday Week 1 -- Week 2 Standards Simmons 2017; ECLL Lamarre: “Lánqīng Guānhuà” Week 3 Lexicon ECLL Masini “Modern Lexicon”; ECLL Duanmu “Word and Wordhood” Week 4 Phonetics and phonology Clark & Yallop 1990: 116-123, 124-132; ECLL Li “Phonemicization of Mandarin” Week 5 Chinese dialect groups F.-K. Li 1937:1-5; Hóu Jīngyī 1989:1-4 Week 6 Dialectal diversity ECLL Handel “Dialect Diversification”; ECLL Norman “Dialect Classification” Week 7 NO MEETING (midterm week) Week 8 Writing system – early development Boltz 1994:143–166 Week 9 Writing system – modern Yú Xiàlóng 1978; Handel 2013 Week 10 THANKSGIVING Week 11 Sino-xenic readings ECLL Osterkamp “Sino-Xenic Readings” A short paper will be due at the end of the quarter, on any topic related to material in the course. Details and suggested topics will be provided at the end of Week 6. Grading breakdown (replaces the breakdown on the Chinese 342 syllabus): • Participation 5% • Reading Responses 5% • Homework 20% • Midterm 15% • Project and presentation 20% • Final examination 20% • Paper 15% All readings (except those marked ECLL) are available in pdf format on the course Canvas site. -

Proquest Dissertations
INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand comer and continuing from left to right in equal sections with small overlaps. Photographs included in the original manuscript have been reproduced xerographically in this copy. Higher quality 6” x 9” black and white photographic prints are available for any photographs or illustrations appearing in this copy for an additional charge. Contact UMI directly to order. Bell & Howell Information and Learning 300 North Zeeb Road, Ann Arbor, Ml 48106-1346 USA 800-521-0600 UMI" ARGUMENT STRUCTURE, HPSG, AND CHINESE GRAMMAR DISSERTATION Presented in Partial Fulfilment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University by Qian Gao, B.A., M.A. ******* The Ohio State University 2001 Dissertation Committee: Approved by Professor Carl J. Pollard, Adviser Professor Peter W. -

Pluralist Universalism
Pluralist Universalism Pluralist Universalism An Asian Americanist Critique of U.S. and Chinese Multiculturalisms WEN JIN The Ohio State University Press | Columbus Copyright © 2012 by The Ohio State University. All rights reserved. Library of Congress Cataloging-in-Publication Data Jin, Wen, 1977– Pluralist universalism : an Asian Americanist critique of U.S. and Chinese multiculturalisms / Wen Jin. p. cm. Includes bibliographical references and index. ISBN 978-0-8142-1187-8 (cloth : alk. paper)—ISBN 978-0-8142-9288-4 (cd) 1. Multiculturalism in literature. 2. Cultural pluralism in literature. 3. Ethnic relations in literature. 4. Cultural pluralism—China. 5. Cultural pluralism—United States. 6. Multicul- turalism—China. 7. Multiculturalism—United States. 8. China—Ethnic relations. 9. United States—Ethnic relations. 10. Kuo, Alexander—Criticism and interpretation. 11. Zhang, Chengzhi, 1948—Criticism and interpretation. 12. Alameddine, Rabih—Criticism and inter- pretation. 13. Yan, Geling—Criticism and interpretation. I. Title. PN56.M8J56 2012 810.9'8951073—dc23 2011044160 Cover design by Mia Risberg Text design by Juliet Williams Type set in Adobe Minion Pro Printed by Thomson-Shore, Inc. The paper used in this publication meets the minimum requirements of the American National Standard for Information Sciences—Permanence of Paper for Printed Library Mate- rials. ANSI Z39.48-1992. 9 8 7 6 5 4 3 2 1 To Jin Yiyu Zhou Huizhu With love and gratitude CONTENTS Preface ix Acknowledgments xv Introduction 1 Chapter 1 Bridging the Chasm: A Survey -

Wen Xue, Ph.D
Wen Xue, Ph.D. RNA Therapeutics Institute University of Massachusetts Medical School 368 Plantation Street, AS4-2053 Worcester, MA 01605 (774) 455-3783 Fax: (508) 856-6696 [email protected] Lab Home page: http://www.umassmed.edu/xuelab/ Education Ph.D., Stony Brook University, The State University of New York, Stony Brook, NY 2004-2009 Thesis: Tumor suppressor gene networks in liver cancer Advisor: Dr. Scott Lowe M.S., Biochemistry, Nanjing University, China 2002-2004 Thesis: Transcriptional regulation in eukaryotes Advisor: Dr. Jin Wang 1998-2002 B.S., Biochemistry, Nanjing University, China Appointments Assistant Professor, RNA Therapeutics Institute, 2014-present Program of Molecular Medicine, and MCCB University of Massachusetts Medical School, Worcester, MA Postdoctoral Research 2009-2014 Koch Institute, MIT, Cambridge, MA Advisor: Dr. Tyler Jacks Honors and Awards NIH Director’s New Innovator Award 2016-2021 American Cancer Society Research Scholars Grant 2016-2020 The Lung Cancer Research Foundation Scientific Merit Award 2015 Worcester Foundation Award 2015 NCI-K99 Pathway to Independence Award 2012-2017 The Leukemia & Lymphoma Society Career Development Program Award 2011-2012 American Association for Cancer Research (AACR) Pre-doctoral Fellowship 2008-2011 Professional Memberships and Activities Member, American Society of Gene & Cell Therapy (ASGCT) 2018-present Member, American Association for Cancer Research (AACR) 2008-present Member, International Liver Cancer Association (ILCA) 2016-present Consultant, Cystic -

A Study of Xu Xu's Ghost Love and Its Three Film Adaptations THESIS
Allegories and Appropriations of the ―Ghost‖: A Study of Xu Xu‘s Ghost Love and Its Three Film Adaptations THESIS Presented in Partial Fulfillment of the Requirements for the Degree Master of Arts in the Graduate School of The Ohio State University By Qin Chen Graduate Program in East Asian Languages and Literatures The Ohio State University 2010 Master's Examination Committee: Kirk Denton, Advisor Patricia Sieber Copyright by Qin Chen 2010 Abstract This thesis is a comparative study of Xu Xu‘s (1908-1980) novella Ghost Love (1937) and three film adaptations made in 1941, 1956 and 1995. As one of the most popular writers during the Republican period, Xu Xu is famous for fiction characterized by a cosmopolitan atmosphere, exoticism, and recounting fantastic encounters. Ghost Love, his first well-known work, presents the traditional narrative of ―a man encountering a female ghost,‖ but also embodies serious psychological, philosophical, and even political meanings. The approach applied to this thesis is semiotic and focuses on how each text reflects the particular reality and ethos of its time. In other words, in analyzing how Xu‘s original text and the three film adaptations present the same ―ghost story,‖ as well as different allegories hidden behind their appropriations of the image of the ―ghost,‖ the thesis seeks to broaden our understanding of the history, society, and culture of some eventful periods in twentieth-century China—prewar Shanghai (Chapter 1), wartime Shanghai (Chapter 2), post-war Hong Kong (Chapter 3) and post-Mao mainland (Chapter 4). ii Dedication To my parents and my husband, Zhang Boying iii Acknowledgments This thesis owes a good deal to the DEALL teachers and mentors who have taught and helped me during the past two years at The Ohio State University, particularly my advisor, Dr.