WO 2017/167716 Al 5 October 2017 (05.10.2017) P O P C T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Membrane Selection Guide
Zaiput Flow Technologies Membranes for Liquid-Liquid Separators Providing in-line liquid-liquid separation for flow chemistry Selection Guide A variety of membranes for your Zaiput separator are available to optimize separation performance and through- put. Membranes are available in both hydrophobic and hydrophilic, they are low cost and easy to replace. Membrane selection process The key parameters for identifying a suitable membrane for separation are the interfacial tension (IFT) between the two phases and the viscosity of the permeating phase (higher viscosities will decrease the throughput of the device). In general, the lower the interfacial tension, the smaller the pore size needs to be. However, smaller pore size reduces the maximum viscosity that can be accommodated by the membrane. A smaller pore size also lowers the total throughput of the device. For general applications, we recommend using a hydrophobic membrane and following the steps below: 1. Identify your mixture’s interfacial tension. Interfacial tension data for a variety of solvent systems is available at the end of this document, in literature or can be searched for online. For complex mixtures, an initial approxima- tion can be obtained by looking at the interfacial tension between the largest component of your system. Please note that salts give a modest increase to the interfacial tension and solvents miscible in both aqueous or organic will decrease it. Often a good estimate is enough, a more accurate value is helpful when dealing with low interfa- cial tension systems (<5 mN/m). 2. Know the viscosity of the permeating phase. Usually an estimate of the viscosity of your permeating phase (organic for a hydrophobic membrane) is enough. -

The Handling, Hazards, and Maintenance of Heavy Liquids Ir"' the Geologic Laboratory
The Handling, Hazards, and Maintenance of Heavy Liquids ir"' The Geologic Laboratory By Phoebe L. Hauff and Joseph Airey GEOLOGICAL SURVEY CIRCULAR 827 1980 United States Department of the Interior CECIL D. ANDRUS, Secretary Geological Survey H. William Menard, Director Free on application to the Branch of Distribution, U.S. Geological Survey, 604 South Pickett Street, Alexandria, VA 22304 CONTENTS Page Abstract • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • . • • • • • • • • • • • • • • • • • • • • • • 1 Introduction • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • 1 Acknowledgments • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • 1 Section I: Properties, hazards, exposure symptoms, and treatment • • • • • • • • • • • • 2 Bromoform • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • 2 Methylene iodide.................................................. 5 Acetone • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • 6 Tetrabromoethane. • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • 8 Clerici solution • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • 9 Section n: Storage, handling, and spills • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • 10 Bromoform...................................................... -

Names & Synonyms Pdf Icon[PDF – 126
V.c. INDEX OF NAMES AND SYNONYMS Example: The references to method title and number are listed in the following manner: ACETIC ACID, 1603 (Method Title) Ethanoic acid, see ACETIC ACID, 1603 (Synonym) Glacial acetic acid, see ACETIC ACID, 1603 (Synonym) Methane carboxylic acid, see ACETIC ACID, 1603 (Synonym) CAS #64-19-7, see ACETIC ACID, 1603 (Chemical Abstracts Registry Number) Acenaphthene, see POLYNUCLEAR AROMATIC HYDROCARBONS, 5506 (HPLC); 5515 (GC) Acenaphthylene, see POLYNUCLEAR AROMATIC HYDROCARBONS, 5506 (HPLC); 5515 (GC) ACETALDEHYDE, 2538 (GC), 3507 (HPLC), see ALDEHYDES, SCREENING, 2539; ALIPHATIC ALDEHYDES, 2018 ACETIC ACID, 1603 Acetic acid anhydride, see ACETIC ANHYDRIDE, 3506 Acetic acid ethyl ester, see ETHYL ACETATE, 1457 Acetic acid butyl ester, see ESTERS I, 1450 Acetic acid 1,1-dimethylethyl ester, see ESTERS I, 1450 Acetic acid ethylene glycol monoethyl ether ester, see ESTERS I, 1450 Acetic acid isobutyl ester, see ESTERS I, 1450 Acetic acid 3-methyl-1-butanol ester, see ESTERS I, 1450 Acetic acid methyl ester, see METHYL ACETATE, 1458 Acetic acid 4-methyl-2-pentanol ester, see ESTERS I, 1450 Acetic acid 1-methyl propyl ester, see ESTERS I, 1450 Acetic acid 1-pentanol ester, see ESTERS I, 1450 Acetic acid 2-pentanol ester, see ESTERS I, 1450 Acetic acid n-propyl ester, see ESTERS I, 1450 Acetic acid vinyl ester, see VINYL ACETATE, 1453 Acetic aldehyde, see ACETALDEHYDE (GC), 2538; 3507 (HPLC) ACETIC ANHYDRIDE, 3506 Acetic ether, see VOLATILE ORGANIC COMPOUNDS (SCREENING), 2549 ACETOIN, 2558 Acetone, see KETONES I, -
C1-C4 Halogenated HC Info 16FEB2018.Xlsx
C1-C4 Halogenated Hydrocarbons/Halocarbons Not Otherwise Listed (C1-C4 NOL) Feb 28, 2018 Administrative Council Mtg - Printed 2/16/20184:11 PM Reported Uses (SOURCES:HAZMAP, NJDHSS, Wikipedia, TSCA CDR, mfr literature) TURA TSCA CDR Chemical CAS/Chemica chemical name chemical fire 2015 Chemical Name Pesti- blowing feedstock / supressant / propell- Inventory? TRI? List l Number (synonyms) formula Solvent refriger-ant etchant Other Tier II cide agent intermed-iate flame ant status* retardant insulation for refrigerators, freezers, commercial refrigeration equipment, refrigerated containers and LNG ships; spray foam insulation; insulated metal panels; slabstock and molded flexible foam; refrigerant for chillers; and solvents for metal cleaning and electronics, and circuit flush. https://www.honeywell-blowingagents.com/?document=solstice-lba-technical- HFO-1233zd (E) brochure&download=1 Hydro-fluoro-olefin C H ClF YYY yhttps://www.honeywell-refrigerants.com/americas/product/solstice-zd/ (HFO) 3 2 3 CDR 2016 Honeywell: 100% as propellants and blowing agents for plastics product mfg; R-1233zd CDR Honeywell Solstice® ZD refrigerant, Solstice® 1233zdE, Solstice Blowing Agent; Arkema (current Forane® 1233zd blowing agent for spray PU foam, appliance insulation, etc. and solvent (may Honeywell not be available in US) product) C1-C4 Cat 102687-65-0 1-Chloro-3,3,3-trifluoropropene Y 106‐95‐6 Allyl bromide 2-Propenyl bromide C3H5Br Y y Manufacture of synthetic perfumes, other allyl compounds; Insecticidal fumigant; 1-Propene, 3-bromo- Chemical intermediate in organic synthesis, for resins (copolymer with sulfur dioxide) and fragrances; As a fumigant (if quite volatile) or as a contact poison; C1-C4 Cat Used in the manufacture of plastics and dyestuff. -

Aldrich Hydrocarbons
Aldrich Hydrocarbons Library Listing – 1,199 spectra Subset of Aldrich FT-IR Library related to hydrocarbons. The Aldrich Material-Specific FT-IR Library collection represents a wide variety of the Aldrich Handbook of Fine Chemicals' most common chemicals divided by similar functional groups. These spectra were assembled from the Aldrich Collection of FT-IR Spectra and the data has been carefully examined and processed by Thermo. The molecular formula, CAS (Chemical Abstracts Services) registry number, when known, and the location number of the printed spectrum in The Aldrich Library of FT-IR Spectra are available. Aldrich Hydrocarbons Index Compound Name Index Compound Name 304 (+)-BETA-PINENE, 98% 483 1,1,2,2-TETRABROMOETHANE, 98% 306 (+)-CAMPHENE, TECH., 80% 481 1,1,2,2-TETRACHLOROETHANE, 235 (+)-CYCLOSATIVENE, 99% 97% 308 (+)-LONGIFOLENE, 98+% 591 1,1,2-TRICHLORO-2,3,3- 289 (-)-ALPHA-CUBEBENE, 97% TRIFLUOROCYCLOBUTANE, 97% 307 (-)-CAMPHENE, 95% 464 1,1,2-TRICHLOROETHANE, 98% 314 (-)-ISOCARYOPHYLLENE, 98% 494 1,1,2- 597 (-)-MENTHYL CHLORIDE, 99+% TRICHLOROTRIFLUOROETHANE, 843 (1,2-DIBROMOETHYL)BENZENE, 99% 99% 566 1,1,3-TRICHLOROPROPENE, 95% 840 (1-BROMOETHYL)BENZENE, 97% 744 1,1,4,4-TETRAPHENYL-1,3- 291 (1R)-(+)-ALPHA-PINENE, 98% (91+% BUTADIENE, SCINTIL-LATION EE/GLC) GRADE, 99+% 287 (1S)-(+)-3-CARENE, 99% 675 1,1-BIS(3,4- 292 (1S)-(-)-ALPHA-PINENE, 98% (81+% DIMETHYLPHENYL)ETHANE, EE/GLC) TECH., 90+% 305 (1S)-(-)-BETA-PINENE, 99% 1156 1,1-BIS(4-CHLOROPHENYL)-2,2,2- 850 (2,2-DICHLORO-1- TRICHLOROETHANE, 99+% METHYLCYCLOPROPYL)BENZENE -

HEAVY LIQUIDS Product Catalog/Technical Guide Letter from the President
HEAVY LIQUIDS Product Catalog/Technical Guide Letter from the President: GeoLiquids is a family business founded in 1950 by my father, Jerome Swimmer. He was a leader in the gemological industry and earned an award from the American Chemical Society for the invention of the process for making Methylene Iodide. My brother Glenn and I carry on the family business the way our father always did, by providing high quality products and outstanding customer service. We take great pride in the fact that many of our customers have been with us for over 50 years. We thank you for your ongoing business and for being part of our family. GeoLiquids supplies heavy liquids worldwide to geologists, research laboratories, university geology departments, gemologists, and mining companies. Our products are available in any quantity starting as small as 1 lb. containers to provide all of our customers with quantities that suit their needs. Our Chicagoland location facilitates easy and quick shipment to all of the United States, Canada, Mexico, and anywhere in the world. We hope this new catalog provides you with the information you need, but if you have any questions, we are always here to help. Please email or call. Sincerely, Mark Swimmer GeoLiquids, Inc [email protected] 800-827-2411 (US and Canada) 847-215-0938 (International) Chemical vs Water Based Products In-stock Shipping Chemical based products used in sink/swim analyses The online ordering system at www.geoliquids.com allows work quickly and cleanly. However, they are toxic and ordering 24/7, 365 days a year. Once an order is placed require care in handling. -

Simple Descriptors for Modeling the Solubility of Gases, Alcohols, and Halogenated Hydrocarbons in Water
Wright State University CORE Scholar Browse all Theses and Dissertations Theses and Dissertations 2007 Simple Descriptors for Modeling the Solubility of Gases, Alcohols, and Halogenated Hydrocarbons in Water Sule Sidigu Wright State University Follow this and additional works at: https://corescholar.libraries.wright.edu/etd_all Part of the Chemistry Commons Repository Citation Sidigu, Sule, "Simple Descriptors for Modeling the Solubility of Gases, Alcohols, and Halogenated Hydrocarbons in Water" (2007). Browse all Theses and Dissertations. 194. https://corescholar.libraries.wright.edu/etd_all/194 This Thesis is brought to you for free and open access by the Theses and Dissertations at CORE Scholar. It has been accepted for inclusion in Browse all Theses and Dissertations by an authorized administrator of CORE Scholar. For more information, please contact [email protected]. SIMPLE DESCRIPTORS FOR MODELING THE SOLUBILITY OF GASES, ALCOHOLS, AND HALOGENATED HYDROCARBONS IN WATER A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science By SULE SIDIGU B.A., Kenyon College, 2003 2007 Wright State University COPYRIGHT BY SULE SIDIGU 2007 WRIGHT STATE UNIVERSITY SCHOOL OF GRADUATE STUDIES September 16, 2007 I HEREBY RECOMMEND THAT THE THESIS PREPARED UNDER MY SUPERVISION BY Sule Sidigu ENTITLED Simple Descriptors for Modeling the Solubility of Gases, Alcohols, and Halogenated Hydrocarbons in Water BE ACCEPTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF Master of Science __________________ Rubin Battino, Ph.D. Thesis Director ___________________ Kenneth Turnbull, Ph.D. Department Chair Committee on Final Examination _________________________ Rubin Battino, Ph.D. _________________________ Paul G. Seybold, Ph.D. _________________________ David A. -
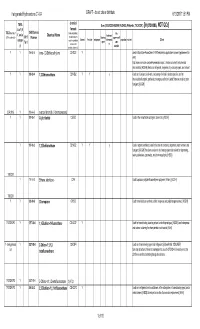
List for Comments C1-C4 Halogenated Hydrocarbons
Halogenated Hydrocarbons C1-C4 DRAFT - do not cite or distribute 6/13/201711:51 PM chemical TURA Uses (SOURCES:HAZMAP, NJDHSS, Wikipedia, TSCA CDR ) [In process, NOT QCd] List? (If formula TSCA Inventory? CAS/Chemica (Yellow highlighting = fire Y, not in Chemical Name feedstock/ (Y/N - student ck) 2015 l Number multiple halogens blowing supressant/fl Solvent Pesticide refrigerant intermedia propellant etchant Other categor could be substituted agent ame Tier II to create other te y) retardant possible substances) Y Y 156-60-5 trans-1,2-Dichloroethylene C2H2Cl2 Y used in MicroCare PowerClean II PW2 electronics applications solvent (replacement for nPB) http://www.microcare.com/p-64-powerclean.aspx; Used as a solvent and chemical intermediate; [ACGIH] Used as a refrigerant, degreaser, dry cleaning agent, and solvent YY 106-93-4 1,2-Dibromoethane C2H4Br2 Y Y y Usedfor perfumes, as a fumigant, adhesives, a solvent, lacquers, a scavenger oils, and forresins; lead [HSDB]in leaded gasoline, and an intermediate in organic synthesis; no longer used in the United States as a soil or grain fumigant; [ACGIH] CDR 2015 Y 106-94-5 n-propyl bromide (1-bromopropane) YY 107-05-1 Allyl chloride C3H5Cl Used in the manufacture of organic chemicals; [ACGIH] Y 107-06-2 1,2-Dichloroethane C2H4Cl2 Y Y y Used in organic synthesis; used in the past as a solvent, degreaser, paint remover, and fumigant; [ACGIH] Has been used as a dry cleaning agent and solvent for degreasing, resins, adhesives, coasmetics, and pharmaceuticals; [HSDB] TRI/CDR Y 116-14-3 Ethene, tetrafluoro- -

(Halogenated Solvents List) and Appendix B
Appendix A Halogenated Solvents List Reference Manual 021002 clean.doc Appendix A Halogenated Solvent List (Alpha Sort) Specific Density Solvent Synonym CAS No. (g/cc) Benzyl chloride Chloromethylbenzene 100-44-7 1.100 Bis(2-chloroethyl)ether Bis(-chloroehtyl)ether 111-44-4 1.220 Bis(2-chloroisopropyl)ether Bis(-chloroisopropyl)ether 108-60-1 1.103 Bromobenzene Phenyl bromide 108-86-1 1.495 Bromochloromehtane Chlorobromomethane 74-97-5 1.934 Bromodichloromethane Dichlorobromomethane 75-27-4 1.980 Bromoethane Ethyl bromide 74-96-4 1.460 Bromoform Tribromomethane 75-25-2 2.890 Carbon tetrachloride Tetrachloromethane 56-23-5 1.594 Chlorobenzene Benzene chloride 108-90-7 1.106 2-Chloroethyl vinyl ether (2-Chlorethoxy)ethene 110-75-8 1.048 Chloroform Trichloromethane 67-66-3 1.483 1-Chloro-1-nitropropane Chloronitropropane 600-25-9 1.209 2-Chlorophenol o-Chlorophenol 95-57-8 1.263 4-Chlorophenyl phenyl ether p-Chlorodiphenyl ether 7005-72-3 1.203 Chloropicrin Trichloronitromethane 76-06-2 1.656 m-Chlorotoluene 108-41-8 1.072 o-Chlorotoluene 2-Chloro-1-methylbenzene 95-45-8 1.082 p-Chlorotoluene 106-43-4 1.066 Dibromochloromethane Chlorodibromomethane 124-48-1 2.451 1,2-Dibromo-3-chlorpropane DPCP 96-12-8 2.050 Dibromodifluoromethane Freon 12-B2 75-61-6 2.297 1,2-Dichlorobenzene o-Dichlorobenzene 95-50-1 1.305 1,3-Dichlorobenzene m-Dichlorobenzene 541-73-1 1.288 1,1-Dichloroethane 1,1-DCA 75-34-3 1.176 1,2-Dichloroethane Ethylene dichloride; 1,2-DCA 107-06-2 1.235 1,1-Dichloroethene Vinylidene chloride; 1,1-DCE 75-35-4 1.218 trans-1,2-Dichloroethene -

Non-Toxic Low-Cost Heavy Liquid Separation in the Geological Survey
11 Non-toxie low-eost heavy liquid separation in the Geological Survey of Greenland Poul Schiøler Density separation of mineral and sediment grains fraction and proceeds to successive1y finer fractions. All into fractions using heavy liquids traditionally employs fractions are separated in the same separation funnel, organic compounds such as bromoform (density 2.89) with the result that thorough deaning between fractions and tetrabromoethane (density 2.96) which are known is not necessary. to be toxic even at very low concentrations (Van Haaf In GGU's palaeontologicallaboratory we use a stan ten, 1969) and possibly carcinogenic. In addition, the dard solution of 2.90 g/cc for the initial separation of a separated grains are washed with organic solvents such sample fraction. Depending on the degree of separation as acetone which may be highly inflammable, and are of the residue, we may choose either to dilute the solu also a health risk. In recent years, a new water soluble tion with a few millilitres of distilled water poured di compound, sodium polytungstate (SPT), rectly into the separation funnel, which is then shaken, 3Na2W04.9W03.H20, has become available as a me or to increase the density of the solution by adding high dium for heavy liquid separations, offering an alterna density SPT solution (3.10 g/cc). This high density solu tive to the heavy organic liquids. Hs use has been dis tion is made up by heating 400 ml of a standard solution cussed by several workers (e.g. Plewinsky & Kamp, (2.90 g/cc) in a 600 ml beaker in the oven at 80°C for 1984; Krukowski, 1988) in a variety of geological set approximately 7 hours, plus cooling time. -
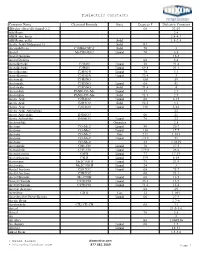
Dielectric Constants
Dielectric Constants Common Name Chemical Formula State Degrees F Dielectric Constant (Ethylenedioxy)diethanol-2,2` 68 23.69 ABS Resin 2.4 ABS Resin, lump 2.4-4.1 ABS Resin, pellet Solid 1.5-2.5 Acatic.Acid (36degrees F) Solid 4.1 Acenaphthene C10H6(CH2)2 Solid 70 3 Acetal MeCH(OEt)2 Liquid 70 3.6 Acetal Bromide 16.5 Acetal Doxime 68 3.4 Acetaldehyde C2H4O Liquid 50 21.8 Acetaldehyde C2H4O Liquid 69.8 21.1 Acetaldoxime C2H5ON Liquid 70 3.4 Acetaldoxime C2H5ON Liquid 73.4 3 Acetamide C3H5NO 180 59 Acetamide C3H5NO Liquid 68 41 Acetamide C3H5NO Solid 71.6 4 Acetanilide PhNH-CO-Me Liquid 71 2.9 Acetanilide PhNH-CO-Me Solid 71.6 2.9 Acetic Acid C2H4O2 Liquid 68 6.15 Acetic Acid C2H4O2 Solid 32.5 4.1 Acetic Acid C2H4O2 Liquid 158 6.62 Acetic Acid, Anhydrous 22 Acetic Anhydride H4H6O3 66 21 Acetic Anhydride H4H6O3 Liquid 70 22 Acetoanilide Granules 2.8 Acetone CO-Me2 Liquid 80 20.7 Acetone CO-Me2 Liquid 130 17.7 Acetone CO-Me2 Gas 212 1.015 Acetone CO-Me2 Liquid -112 31 Acetone CO-Me2 32 1.0159 Acetonitrile CH3-CN Liquid 70 37.5 Acetonitrile CH3-CN Liquid 179.6 26.6 Acetophenone C8H8 Liquid 77 17.39 Acetophenone C8H8 Liquid 394 8.64 Acetoxime Me2C:NOH Liquid 75 23.9 Acetoxime Me2C:NOH Liquid 24 3 Acetyl Acetone C5H2O2 Liquid 68 25.7 Acetyl Acetone C5H2O2 68 23.1 Acetyl Bromide Me-COBr Liquid 68 16.5 Acetyl Chloride C2H3ClO Liquid 35.6 16.9 Acetyl Chloride C2H3ClO Liquid 71.6 15.8 Acetyle Acetone 68 25 Acetylene C2H2 Gas 32 1.001 Acetylmethyl Hexyl Ketone Liquid 66 27.9 Acrylic Resin 2.7-6 Acrylonitrile CH2:CH-CN 68 33 Acteal 21.0-3.6 Acteal