Table 4: Protective Action Criteria (PAC) Rev 27 Based on Applicable 60-Minute Aegls, Erpgs, Or Teels
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Theoretical Study of Sarin Adsorption On
Chemical Physics Letters 738 (2020) 136816 Contents lists available at ScienceDirect Chemical Physics Letters journal homepage: www.elsevier.com/locate/cplett Research paper Theoretical study of sarin adsorption on (12,0) boron nitride nanotube doped with silicon atoms T ⁎ ⁎ Jeziel Rodrigues dos Santosa, , Elson Longo da Silvab, Osmair Vital de Oliveirac, , José Divino dos Santosa a Universidade Estadual de Goiás, Campus Anápolis, CEP: 75.132-903 GO, Brazil b INCTMN, LIEC, Departamento de Química da Universidade Federal de São Carlos, CEP: 13.565-905 São Carlos, SP, Brazil c Instituto Federal de Educação, Ciência e Tecnologia de São Paulo, Campus Catanduva, CEP: 15.808-305 Catanduva, SP, Brazil HIGHLIGHTS • DFT method was used to study the adsorption of nerve agent sarin by BNNT. • Electronic properties of pristine BNNT are improved by Si impurity atoms. • The adsorption of sarin by Si-doped BNNT is highest favorable than the pure BNNT. • Si-doped BNNT can be a new gas sensor for sarin gas detection and its derivatives. ARTICLE INFO ABSTRACT Keywords: Sarin gas is one of the most lethal nerve agent used in chemical warfare, which its detection is import to prevent Nerve agent sarin a chemical attack and to identify a contamination area. Herein, density functional theory was used to investigate Gas sensor the (12,0) boron nitride nanotube (BNNT) and Si–doped BNNT as possible candidates to sarin detection. The Si- Boron nitride nanotube atoms doped improve the electronic properties of nanotubes by altering the electrostatic potential, HOMO and DFT LUMO energies. Based in the adsorption energies and the conductivity increased to ~33 and 350%, respectively, for Si- and 2Si-BNNT imply that they can be used for sarin detection. -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Spectrophotometric Determination of Thiocyanate In
Investigative Forensic Sciences © All rights are reserved by Buddha D. Paul and Thomas Research Article Open Access Spectrophotometric Determination of Thiocyanate in Human Saliva by a Unique Iodine-Azide-Chromogenic Substrate Reaction and its Application in Distinguishing Tobacco Smokers from Non-Smokers† Buddha D. Paul and Thomas Bosy Division of Forensic Toxicology, Office of the Armed Forces Medical Examiner, Dover AFB, Delaware, USA. Abstract A method to detect thiocyanate (SCN) in human saliva is presented. Thiocyanate concentrations appear to be diagnostic when classifying smokers or non-smokers, and in determining some clinical conditions. The method involves the reaction of SCN with excess iodine and azide, and spectroscopic detection of unreacted iodine by a chromogenic substrate, ABTS. The calibration was linear over the range of 12.5-150 µmol/L (slope = 0.0086 delta-Abs/SCN µmol/L, intercept = -0.0160 delta-Abs, R2 0.9998). The method was applied to analyze 29 saliva specimens. The results were similar to those obtained from a gas chromatography-mass 2 spectrometry method (slope = 0.9595, R 0.9790). Based on Grubbs equation applied to specimens from non-smoking subjects, a threshold concentration of 1100 µmol/L for SCN was determined to distinguish smokers from the non-smokers. The SCN concentrations in 18 out of 20 saliva specimens collected from 2 smokers were above this threshold. The specimens from smokers were also examined for nicotine and cotinine by a GCMS method. While nicotine concentrations were found to vary, the cotinine concentrations remained stable, 134+29 ng/mL. Generally, the presence of nicotine/cotinine in specimens only indicates exposure to tobacco products, but the presence of any of these compounds with elevated SCN, is an indication of smoking. -

Calarp) Program
California Accidental Release Prevention (CalARP) Program Administering Agency Guidance January 31, 2005 Preface This document provides general guidance to help Administering Agencies (AAs) implement and enforce the California Accidental Release Prevention (CalARP) Program. The intent is to identify the elements of the Program applicable to each regulated business, and assist AAs with oversight of the CalARP Program statutes and regulations. This document is not a substitute for the CalARP Program regulations; it does not impose legally binding requirements. About This Document This document follows the format of the California Code of Regulations, Title 19, Division 2, Chapter 4.5: California Accidental Release Prevention (CalARP) Program. The regulatory sections are presented in parentheses for ease of reference. Acknowledgements The California Emergency Management Agency (Cal EMA) would like to thank the following people for their valuable assistance in the preparation of this document: Howard Wines, Hazardous Materials Specialist, City of Bakersfield Fire Department Robert Distaso P.E., Fire Safety Engineer, Orange County Fire Authority Randall L. Sawyer, Supervisor, Accidental Release Prevention Programs, Contra Costa County Health Services Department Beronia Beniamine, Senior Hazardous Materials Specialist, Stanislaus County Environmental Resources Department Angie Proboszcz, Risk Management Program Coordinator, USEPA Region 9 Jon Christenson, Senior Environmental Health Specialist, Merced County Department of Public Health Teresa -

Chloroplatinic Acid Hydrate
Chloroplatinic acid hydrate sc-239532 Material Safety Data Sheet Hazard Alert Code Key: EXTREME HIGH MODERATE LOW Section 1 - CHEMICAL PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME Chloroplatinic acid hydrate STATEMENT OF HAZARDOUS NATURE CONSIDERED A HAZARDOUS SUBSTANCE ACCORDING TO OSHA 29 CFR 1910.1200. NFPA FLAMMABILITY0 HEALTH3 HAZARD INSTABILITY0 SUPPLIER Santa Cruz Biotechnology, Inc. 2145 Delaware Avenue Santa Cruz, California 95060 800.457.3801 or 831.457.3800 EMERGENCY: ChemWatch Within the US & Canada: 877-715-9305 Outside the US & Canada: +800 2436 2255 (1-800-CHEMCALL) or call +613 9573 3112 SYNONYMS Cl6-H2-Pt, H2PtCl6, "chloroplatinic (IV) acid", "chloroplatinic acid", "chlorplatinic acid", "dihydrogen hexachloroplatinate", "hydrogen hexachlorplatinate", "platinum chloride", "acid platinic chloride" Section 2 - HAZARDS IDENTIFICATION CHEMWATCH HAZARD RATINGS Min Max Flammability: 0 Toxicity: 3 Body Contact: 3 Min/Nil=0 Low=1 Reactivity: 0 Moderate=2 High=3 Chronic: 2 Extreme=4 CANADIAN WHMIS SYMBOLS 1 of 9 EMERGENCY OVERVIEW RISK Toxic if swallowed. Causes burns. Risk of serious damage to eyes. May cause SENSITISATION by inhalation and skin contact. POTENTIAL HEALTH EFFECTS ACUTE HEALTH EFFECTS SWALLOWED ! Toxic effects may result from the accidental ingestion of the material; animal experiments indicate that ingestion of less than 40 gram may be fatal or may produce serious damage to the health of the individual. ! The material can produce chemical burns within the oral cavity and gastrointestinal tract following ingestion. ! Ingestion of acidic corrosives may produce burns around and in the mouth. the throat and esophagus. EYE ! The material can produce chemical burns to the eye following direct contact. Vapors or mists may be extremely irritating. -

A Study of Hydrogen Exchange in Benzene And
A STUDY OF HYDROGEN EXCHANGE IN BENZENE AND SEVERAL ALKYLBENZENES By REX LYNN ELMORE B~chelor of Arts Austin College Sherman, Texas 1960 Submitted to the faculty of the Graduate School of the Oklahpma State University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY August, 1965 J OKLAHOMA Jjf STATE UNIVERSITY .;.;, i' LIBRARY f DEC 6 1965 j' A STUDY OF HYDROGEN EXCHANGE IN BENZE~ AND ,,:.-· ;" . ·..... : SEVERAL ALKYLBENZENES f ~~~~~~~"'~"''""i;·~l'J!'~~ Thesis Approved: 593417 ii .ACKNOWLEDGMENTS Grateful acknowledgment is made to-Dr. E. M. Hodnett, research adviser, for his patience, guidance, and assistance throughout this work; to Dr. O. C. Dermer, head of the Chemistry Department, for his reading of the manuscript prior to its being typed; to Mr. C.R. Williams, formerly in the Chemistry Department, for his assistance in the early phases of the computer programming; to Mr. Preston Gant, Central Research Division of the Continental Oil Company, for helpful suggestions on the tritium assays; and to Mr. Prem S. Juneja for his helpful suggestions concerning experimental details which arose from time to time. Acknowledgment is made of financial assistance in the form of a graduate teaching assistantship in the Chemis.try Department. Also, a research as.sistantship from the Atomic Energy Commission, Contract No. AT(ll-1)-1049, through the Research Foundation is appreciated. Research done during the term of the latter assistantship provided material for this thesis. iii TABLE OF CONTENTS Chapter Page I. INTRODUCTION. 1 II. HISTORICAL, , 3 III. INTRODUCTION TO THE EXPERIMENTAL WORK 14 Objectives and Plan of the Study. -

Specialty Direct Supply Drug List
DRAFT SPECIALTY DIRECT SUPPLY DRUG LIST (DSDL) 9/27/2005 Drug Description Effective Date 5-HT3 Receptor Antagonists ALOXI - SOLN 12/09/2004 ANZEMET - SOLN 12/09/2004 ANZEMET - TABS 12/09/2004 KYTRIL - SOLN 12/09/2004 KYTRIL - TABS 12/09/2004 Additional Solids ALPROSTADIL - POWD 12/09/2004 PROSTAGLANDIN E1 - POWD 12/09/2004 Agents for Gaucher Disease CEREZYME - SOLR 12/09/2004 Agents for Pheochromocytoma PHENTOLAMINE MESYLATE - SOLR 12/09/2004 REGITINE - SOLR 12/09/2004 Alkylating Agents CARBOPLATIN - SOLN 12/09/2004 CARBOPLATIN - SOLR 12/09/2004 CISPLATIN - SOLN 12/09/2004 CISPLATIN AQ - SOLN 12/09/2004 ELOXATIN - SOLR 12/09/2004 MYLERAN - TABS 12/09/2004 PARAPLATIN - SOLN 12/09/2004 PARAPLATIN - SOLR 12/09/2004 PLATINOL - SOLN 12/09/2004 PLATINOL AQ - SOLN 12/09/2004 THIOPLEX - SOLR 12/09/2004 THIOTEPA - SOLR 12/09/2004 Alpha-Proteinase Inhibitor (Human) ARALAST - SUSR 07/01/2005 PROLASTIN - SUSR 07/01/2005 ZEMAIRA - SOLR 07/01/2005 AMINOGLYCOSIDES APOGEN - SOLN 12/09/2004 GARAMYCIN - SOLN 12/09/2004 GENTAMICIN SULFATE - SOLN 12/09/2004 G-MYCIN - SOLN 12/09/2004 JENAMICIN - SOLN 12/09/2004 NEBCIN - SOLN 12/09/2004 NEBCIN MDV - SOLN 12/09/2004 STORZ-G - SOLN 12/09/2004 TOBI - NEBU 12/09/2004 TOBRAMYCIN SULFATE - SOLN 12/09/2004 TOBRAMYCIN SULFATE FLIPTO - SOLN 12/09/2004 Antianxiety Agents - Misc. rev A Page 1 of 13 MaineCare Direct Supply Drug List (DSDL) Drug Description Effective Date Antianxiety Agents - Misc. - Continued - DROPERIDOL - POWD 12/09/2004 DROPERIDOL - SOLN 12/09/2004 INAPSINE - SOLN 12/09/2004 Antiarrhythmics Type III -

Laxatives for the Management of Constipation in People Receiving Palliative Care (Review)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UCL Discovery Laxatives for the management of constipation in people receiving palliative care (Review) Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2015, Issue 5 http://www.thecochranelibrary.com Laxatives for the management of constipation in people receiving palliative care (Review) Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 BACKGROUND .................................... 2 OBJECTIVES ..................................... 4 METHODS ...................................... 4 RESULTS....................................... 7 Figure1. ..................................... 8 Figure2. ..................................... 9 Figure3. ..................................... 10 DISCUSSION ..................................... 13 AUTHORS’CONCLUSIONS . 14 ACKNOWLEDGEMENTS . 14 REFERENCES ..................................... 15 CHARACTERISTICSOFSTUDIES . 17 DATAANDANALYSES. 26 ADDITIONALTABLES. 26 APPENDICES ..................................... 28 WHAT’SNEW..................................... 35 HISTORY....................................... 35 CONTRIBUTIONSOFAUTHORS . 36 DECLARATIONSOFINTEREST . 36 SOURCESOFSUPPORT . 36 DIFFERENCES -

United States Patent (19) 11 Patent Number: 4,820,674 Shiozawa Et Al
United States Patent (19) 11 Patent Number: 4,820,674 Shiozawa et al. 45) Date of Patent: Apr. 11, 1989 54 PROCESS FOR PREPARING A 58) Field of Search ....................... 502/169, 172, 230; HYDROSLYLATION CATALYST 556/479, 136; 549/206, 211 75 Inventors: Kouji Shiozawa, Saitama; Yoshiharu (56) References Cited Okumura, Tokyo; Chihiro Imai, U.S. PATENT DOCUMENTS Kanagawa; Nobukazu Okamoto, 3,220,972 11/1965 Lamoreaux ......................... 502/169 Saitama, all of Japan 3,624,119 11/1971 Rothe.................................. 502/169 73) Assignee: Toa Nenryo Kogyo Kabushiki Kaisha, 3,814,731 6/1974 Nitzsche .............................. 502/169 Tokyo, Japan Primary Examiner-Patrick P. Garvin Assistant Examiner-Elizabeth Irzinski 21 Appl. No.: 149,092 Attorney, Agent, or Firm-Wenderoth, Lind and Ponack 22 Filed: Jan. 27, 1988 57 ABSTRACT A process for preparing a hydrosilylation catalyst by 30 Foreign Application Priority Data dissolving chloroplatinic acid H2PtCl6 in a cyclic ether or cyclic ester containing at least 3 carbon atoms, and Jan. 29, 1987 JP Japan .................................. 62-18916 maintaining the resulting solution at a temperature of at (51 Int. Cl. .............................................. B01J 31/00 least 3O C. 52 U.S. Cl. .................................... 502/169; 502/172; 502/230 4. Claims, No Drawings 4,820,674 1. 2 the resulting solution at a temperature of at least 30 C., PROCESS FOR PREPARING A preferably from 50° to 120° C. HYDROSILYLATION CATALYST DETAILED DESCRIPTION OF THE BACKGROUND OF THE INVENTION INVENTION This invention relates to a process for preparing a A process for preparing a hydrosilylation catalyst hydrosilylation catalyst which is preferably used in according to the present invention will be fully de synthesizing a silane coupling agent and, more particu scribed hereinafter. -
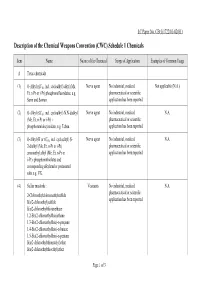
Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals
LC Paper No. CB(1)1722/01-02(01) Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals Item Name Nature of the Chemical Scope of Application Examples of Common Usage A Toxic chemicals (1) O-Alkyl (≤C10, incl. cycloalkyl) alkyl (Me, Nerve agent No industrial, medical, Not applicable (N.A.) Et, n-Pr or i-Pr) phosphonofluoridates, e.g. pharmaceutical or scientific Sarin and Soman. application has been reported. (2) O-Alkyl (≤C10, incl. cycloalkyl) N,N-dialkyl Nerve agent No industrial, medical, N.A. (Me, Et, n-Pr or i-Pr) - pharmaceutical or scientific phosphoramidocyanidate, e.g. Tabun. application has been reported. (3) O-Alkyl (H or ≤C10, incl. cycloalkyl) S- Nerve agent No industrial, medical, N.A. 2-dialkyl (Me, Et, n-Pr or i-Pr) pharmaceutical or scientific aminoethyl alkyl (Me, Et, n-Pr or application has been reported. i-Pr)- phosphonothiolates and corresponding alkylated or protonated salts e.g. VX. (4) Sulfur mustards : Vesicants No industrial, medical, N.A. pharmaceutical or scientific 2-Chloroethylchloromethylsulfide application has been reported. Bis(2-chloroethyl)sulfide Bis(2-chloroethylthio)methane 1,2-Bis(2-chloroethylthio)ethane 1,3-Bis(2-chloroethylthio)-n-propane 1,4-Bis(2-chloroethylthio)-n-butane 1,5-Bis(2-chloroethylthio)-n-pentane Bis(2-chloroethylthiomethyl)ether Bis(2-chloroethylthioethyl)ether Page 1 of 3 Item Name Nature of the Chemical Scope of Application Examples of Common Usage (5) Lewisites : Vesicants No industrial, medical, N.A. pharmaceutical or scientific Lewisite 1 : 2-Chlorovinyldichloroarsine application has been reported. Lewisite 2 : Bis(2-chlorovinyl)chloroarsine Lewisite 3 : Tris(2-chlorovinyl)arsine (6) Nitrogen mustards : Vesicants The chemical has medical Only HN2 has been reported to application. -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -

1,45%,562 UNITED SATES P All.‘ Bl If" Til?
Patented May 1, 1923. 1,45%,562 UNITED SATES P All.‘ bl if" til? . ROBERT E. WILSON, LEON ‘JV. PARSONS, AND STANLEY 1E. OHXSHOLIYI, OF WASHINGTON, DISTRICT OF COLUMBIA. PROCESS THE PRODUCTION OE‘ All‘HALLEAETELMLETAL PERTJIANGANATES. N0 Drawing". Application ?led September 27, 1918. Serial No. 255,975. To (all to]: am it may concern. .' manganate by oxidation or acidification, Be it known that we, Romain‘ E. lllinsou, metatheses into calcium pern'iangamite by LEON “7. Parsons, and STANLEY L. Unis treatment With calcium sulphate or milk at HoLM, citizens of the United States. and sta lime. tioned at ViTashington, District of Columbia, O'li' these four possible methods, (1.) is not 60 in the o?icc of the Director the Chemical a possible large scale method. on account l/Varfare Service, Research Division, have in of its use ot silver; (2) and are elec vented a Process for the ll’roduction oif Al trolytic methods Without a. great deal out kali-Earth-l\letal Permanpjanates, of which promise, and are to be considered elsewhere; ll) the ‘following is a speci?cation. (ll) the principal subject of this applica G3 in The present invention relates to the pro tion. duction oi? alkah» earth metal permangam Three distinct methods for preparing: ba~ nates and especially the permanganates of rium (or strontium) manganate have been calcium and magnesium as these have beenv here investigated. The ?rst of? these meth found to be very ellicient oxidizing agents ods involves heating together barium perox 70 for certain purposes, more e?icient even. than ide, hydroxide, or a salt, such as the nitrate the permanganates of the allmliearth metals.