Spectrophotometric Determination of Thiocyanate In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Calarp) Program
California Accidental Release Prevention (CalARP) Program Administering Agency Guidance January 31, 2005 Preface This document provides general guidance to help Administering Agencies (AAs) implement and enforce the California Accidental Release Prevention (CalARP) Program. The intent is to identify the elements of the Program applicable to each regulated business, and assist AAs with oversight of the CalARP Program statutes and regulations. This document is not a substitute for the CalARP Program regulations; it does not impose legally binding requirements. About This Document This document follows the format of the California Code of Regulations, Title 19, Division 2, Chapter 4.5: California Accidental Release Prevention (CalARP) Program. The regulatory sections are presented in parentheses for ease of reference. Acknowledgements The California Emergency Management Agency (Cal EMA) would like to thank the following people for their valuable assistance in the preparation of this document: Howard Wines, Hazardous Materials Specialist, City of Bakersfield Fire Department Robert Distaso P.E., Fire Safety Engineer, Orange County Fire Authority Randall L. Sawyer, Supervisor, Accidental Release Prevention Programs, Contra Costa County Health Services Department Beronia Beniamine, Senior Hazardous Materials Specialist, Stanislaus County Environmental Resources Department Angie Proboszcz, Risk Management Program Coordinator, USEPA Region 9 Jon Christenson, Senior Environmental Health Specialist, Merced County Department of Public Health Teresa -

Solubility and Solution-Phase Chemistry of Isocyanic Acid, Methyl Isocyanate, 2 and Cyanogen Halides 3 4 5 6 James M
Atmos. Chem. Phys. Discuss., https://doi.org/10.5194/acp-2018-1160 Manuscript under review for journal Atmos. Chem. Phys. Discussion started: 9 November 2018 c Author(s) 2018. CC BY 4.0 License. 1 Solubility and Solution-phase Chemistry of Isocyanic Acid, Methyl Isocyanate, 2 and Cyanogen Halides 3 4 5 6 James M. Roberts1, and Yong Liu2 7 8 1. NOAA/ESRL Chemical Sciences Division, Boulder, Colorado, 80305 9 2. Department of Chemistry, University of Colorado, Denver, Denver, Colorado, 80217 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 Atmos. Chem. Phys. Discuss., https://doi.org/10.5194/acp-2018-1160 Manuscript under review for journal Atmos. Chem. Phys. Discussion started: 9 November 2018 c Author(s) 2018. CC BY 4.0 License. 52 Abstract 53 54 Condensed phase uptake and reaction are important atmospheric removal processes for reduced nitrogen 55 species, isocyanic acid (HNCO), methyl isocyanate (CH3NCO) and cyanogen halides (XCN, X =Cl, Br, I), yet many 56 of the fundamental quantities that govern this chemistry have not been measured or are understudied. Solubilities 57 and first-order reaction rates of these species were measured for a variety of solutions using a bubble flow reactor 58 method with total reactive nitrogen (Nr) detection. The aqueous solubility of HNCO was measured as a function of 59 pH, and exhibited the classic behavior of a weak acid, with an intrinsic Henry’s law solubility of 20 (±2) M/atm, and -4 60 a Ka of 2.0 (±0.28) ×10 M (which corresponds to pKa = 3.7 ±0.06) at 298K. -
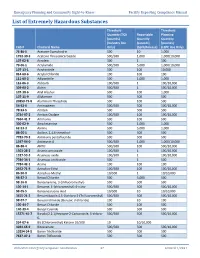
List of Extremely Hazardous Substances
Emergency Planning and Community Right-to-Know Facility Reporting Compliance Manual List of Extremely Hazardous Substances Threshold Threshold Quantity (TQ) Reportable Planning (pounds) Quantity Quantity (Industry Use (pounds) (pounds) CAS # Chemical Name Only) (Spill/Release) (LEPC Use Only) 75-86-5 Acetone Cyanohydrin 500 10 1,000 1752-30-3 Acetone Thiosemicarbazide 500/500 1,000 1,000/10,000 107-02-8 Acrolein 500 1 500 79-06-1 Acrylamide 500/500 5,000 1,000/10,000 107-13-1 Acrylonitrile 500 100 10,000 814-68-6 Acrylyl Chloride 100 100 100 111-69-3 Adiponitrile 500 1,000 1,000 116-06-3 Aldicarb 100/500 1 100/10,000 309-00-2 Aldrin 500/500 1 500/10,000 107-18-6 Allyl Alcohol 500 100 1,000 107-11-9 Allylamine 500 500 500 20859-73-8 Aluminum Phosphide 500 100 500 54-62-6 Aminopterin 500/500 500 500/10,000 78-53-5 Amiton 500 500 500 3734-97-2 Amiton Oxalate 100/500 100 100/10,000 7664-41-7 Ammonia 500 100 500 300-62-9 Amphetamine 500 1,000 1,000 62-53-3 Aniline 500 5,000 1,000 88-05-1 Aniline, 2,4,6-trimethyl- 500 500 500 7783-70-2 Antimony pentafluoride 500 500 500 1397-94-0 Antimycin A 500/500 1,000 1,000/10,000 86-88-4 ANTU 500/500 100 500/10,000 1303-28-2 Arsenic pentoxide 100/500 1 100/10,000 1327-53-3 Arsenous oxide 100/500 1 100/10,000 7784-34-1 Arsenous trichloride 500 1 500 7784-42-1 Arsine 100 100 100 2642-71-9 Azinphos-Ethyl 100/500 100 100/10,000 86-50-0 Azinphos-Methyl 10/500 1 10/10,000 98-87-3 Benzal Chloride 500 5,000 500 98-16-8 Benzenamine, 3-(trifluoromethyl)- 500 500 500 100-14-1 Benzene, 1-(chloromethyl)-4-nitro- 500/500 -

On the Cyanogen Halides by P
ON THE CYANOGEN HALIDES BY P. KAILASAM (From the Chemistry Department, Madras Christian College) Received July 31, 1941 (Communicated by Sir C. V. Raman, xt., F.R.S., NN.L.) I. Action of Halogens on Cyanogen Halides No observations appear to have been recorded on the action of halogens on cyanogen halides except that of chlorine on cyanogen chloride (Wurtz, 1851; Naumann and Vogt, 1870) and bromine on cyanogen bromide in ether (Poonamarew, 1885) which polymerise them to cyanuric chloride and cyanuric bromide respectively. The object of the present investigation is to study the possible displacement reactions with the cyanogen halides. The action of chlorine gas on cyanogen iodide and the action of bromine on cyanogen chloride were first studied. It was expected that chlorine would displace iodine from cyanogen iodide forming cyanogen chloride, but it was found that cyanogen iodide was not affected by dry chlorine. Dry cyanogen iodide, prepared by warming an intimate mixture of one part of iodine and two parts of AgCN, at about 40°, was sublimed into a narrow tube which could be heated in a water-bath. Dry chlorine was passed through the tube for nearly two hours. No immediate reaction occurred, and even after leaving for a week at room temperature (30°) the white needles of cyanogen iodide remained unchanged. The tube was then slowly heated, but no reaction occurred up to the sublimation temperature of cyanogen iodide (about 40°). It was found that cyanogen iodide could be sublimed unchanged in an atmosphere of dry chlorine. Since chlorine does not displace iodine from cyanogen iodide, it was thought that bromine might displace chlorine from cyanogen chloride form- ing cyanogen bromide. -

List of Lists
United States Office of Solid Waste EPA 550-B-10-001 Environmental Protection and Emergency Response May 2010 Agency www.epa.gov/emergencies LIST OF LISTS Consolidated List of Chemicals Subject to the Emergency Planning and Community Right- To-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act • EPCRA Section 302 Extremely Hazardous Substances • CERCLA Hazardous Substances • EPCRA Section 313 Toxic Chemicals • CAA 112(r) Regulated Chemicals For Accidental Release Prevention Office of Emergency Management This page intentionally left blank. TABLE OF CONTENTS Page Introduction................................................................................................................................................ i List of Lists – Conslidated List of Chemicals (by CAS #) Subject to the Emergency Planning and Community Right-to-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act ................................................. 1 Appendix A: Alphabetical Listing of Consolidated List ..................................................................... A-1 Appendix B: Radionuclides Listed Under CERCLA .......................................................................... B-1 Appendix C: RCRA Waste Streams and Unlisted Hazardous Wastes................................................ C-1 This page intentionally left blank. LIST OF LISTS Consolidated List of Chemicals -

Zeitschrift Für Naturforschung / B / 48 (1993)
1305 Notizen On the Synthesis of D-Homoandrostanes Treatment of 2 with cyanogen azide, followed by hydrolysis led to 3/?-hydroxy-D-homo-5 a-an- M. Pelecanou* and S. Nicolaropoulos drostan-17 a-one (3) in 32% yield. Other minor Laboratory of Pharmaceutical Chemistry, products that were isolated did not amount to more School of Health Sciences, University of Patras, 26500 Patras, Greece than 4%. Even though the yield of the expansion reaction Z. Naturforsch. 48 b, 1305-1306 (1993); received April 2,1993 is low, the observed migrational selectivity (sec ondary vs. tertiary D-ring bond) makes the above D-Homoandrostanes, Epiandrosterone, reaction scheme a useful way to D-homo-epiand- Wittig Reaction, Cyanogen Azide, D-Ring Expansion rosterone. It should be noted that a great number of unsymmetrical ketones that have been subjected The D-ring expansion of 3 /?-hydroxy-5 a- to the cyanogen azide ring-expansion reaction do androstan-17-one (epiandrosterone) by the cy anogen azide ring-expansion reaction is describ not display any significant migrational selectivity ed. Epiandrosterone was first converted to 17-me- [4]- thylene-3 yS-hydroxy-5 a-androstane by a modifi cation of the Wittig reaction employing methyl- Experimental sulfinyl carbanion-dimethyl sulfoxide. Treatment Melting points were determined on a Fisher Johns of the 17-methyleno derivative with cyanogen azide followed by hydrolysis led to 3 /3-hydroxy- melting point apparatus and are uncorrected. IR D-homo-5 a-androstan-17 a-one with migrational spectra were recorded on a Perkin-Elmer 521 spec selectivity. trometer. NMR data were obtained in CDC1 3 with a Bruker AC 200 E spectrometer equipped with an Preparation of D -homologs of androstan-17-ones Aspect 3000 computer. -

Chemical Hygiene Plan Ii Revised 03/2021 Table of Contents
MAR 2021 Office of Environmental Health and Safety Principal Author/Editor: David Webber, PhD/Chemical Hygiene Officer Contributing Authors/Editors: Nikolai Evdokimov, PhD, James Gibson, PhD, Tania Guardado, PhD, Amanda Jevons, Deona Willes, MPH Graphics/Design: Alfred M. Bouziane, MS, Brent Pantell USC Chemical Hygiene Plan ii Revised 03/2021 Table of Contents i.0 2021 Revision Summary Section 3.0 vi Section 4.0 vi Section 5.0 vii Section 7.0 vii Section 8.0 viii Section 10.0 x Appendix D x Appendix G x 1.0 Introduction Purpose and Scope 1.1 Sources of Safety Information 1.2 2.0 Regulatory Requirements 3.0 Roles and Responsibilities Research Safety Oversight Committee (RSOC) 3.1 Campus-Wide Chemical Safety Committee (CCSC) 3.1 Other Safety Committees 3.2 Office of Environmental Health & Safety 3.2 Principal Investigator (PI) 3.3 Training Requirements 3.5 4.0 Basics of Laboratory Safety Hazard, Risk, and Safety Management 4.1 Hierarchy of Safety Controls 4.1 Group Safety Management and Safety Culture 4.4 USC Chemical Hygiene Plan iii Revised 03/2021 Basics of Lab Facilities, Equipment, and Emergency Supplies 4.7 Emergency Equipment and Supplies 4.15 Open Flames 4.20 5.0 Hazard Communication Labeling and Signage Systems 5.2 Labelling and Signage in the Lab: What You Need to Do 5.5 Safety Data Sheets (SDSs): What Are They? 5.7 SDSs in The Lab: What You Need to Do 5.8 6.0 Hazardous Chemicals and Hazard Classification Introduction 6.1 Health-Hazardous Chemicals: Routes of Exposure 6.2 Particularly Hazardous Substances (PHS) 6.16 7.0 Chemical -

The Analysis of Cyanide Silver-Plating Solutions
RP384 THE ANALYSIS OF CYANIDE SILVER-PLATING SOLUTIONS By R. M. Wick ABSTRACT Methods for the analysis of cyanide silver-plating solutions were studied, including the determination of free cyanide, total cyanide, carbonate, chloride, ammonia, silver, iron, copper, and mercury. Electrometric titrations showed that the Liebig method for alkali cyanide is correct to better than 0.2 per cent. Addition of iodide makes the method still more accurate and overcomes the effects of impurities on the titration. The nonagreement of the Liebig and Hannay methods was studied by electro- metric titrations, which showed that the visual Hannay method gives high results although the electrometric Hannay titration is accurate. The determination of total cyanide was accomplished by distillation with sulphuric acid, and of total effective cyanide by titration with iodine. Silver may be separated as silver sulphide, by precipitation with zinc, or by decomposition with acid, after which a determination by any standard method is applicable. Carbonate may be determined by precipitating and filtering out barium car- bonate and titrating it with acid or by titrating a sample with acid after adding silver nitrate to combine with the free cyanide. The usual method for the determination of chloride involves prolonged treat- ment with nitric acid, which converts the precipitated silver cyanide to silver chloride. A new method for separating chloride was investigated in which the silver is precipitated as sulphide and the iron is removed by precipitation as manganous ferrocyanide. The chloride in the filtrate can be determined by the usual methods. Iron and copper can be determined by the regular analytical methods after decomposition of the plating solution with sulphuric acid. -

Us. Department of Commerce National Bureau of Standards National Bureau of Standards Report
c' c' I s NATIOMAL BUREAU ,OF' STANDARDS REPORT 86 91 MATRlX ISOLATION STUDY OF THE PHOTOLYSXS OF CYANO- AZIDE, T€B INFR&ED AND ULTRAVIOLET SPECTRA OF TRIE RADXCAL NCN* by Dolphus E, Milligaa, Marilyn E, J~CQPL,;and Araold M, Base Technical Report to Natiunal Aeronautics and Space Administration Waehfngton, D. C, GPO PRICE $ t: CFSTI PRICE(S) $ &- LY I, Hard copy (HC) S.fQ Microfiche (MF) I Tt ff 653 July 65 US. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS NATIONAL BUREAU OF STANDARDS REPORT NBS PROJECT NBS REPORT 221- 11-2210461 May 18, 1965 8691 - MATRIX ISOLATION STUDY GF THE PhOTOLYSIS OF G‘:A,IOGEN AZIDE. THE INFRARED AND ULTRAVIOLET SPECTRA OF THE lXEE 2ADLCAL KCN, bY Arnold M. Bass Technical Kepor t to National Aeronautics and Space Administration PJashington, D. C. EASA Order No, R-64 IMPORlANT NOTICE NATIONAL BUREAU OF STA TS are usually preliminary or progress accounting documents intended for use within the Government. Before ports is formally published it is subjected to additional evaluation and review. For this reaso re listing of this Report, either in whole or in part, is not a the Office of the Director, National Bureau of Standards, Was , by the Government agency for which the Report has been specif a1 copies for its own use. U. S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS I- - Preface The work described in this report wa8 done by the author in collaboration with Drs. Milligan and Jacax. The latter performed the measurements and analysis of the infrared data. The author of this report carried Out the ultraviolet mea- surementa. -

Massachusetts Oil and Hazardous Material List
SUBPART P: MASSACHUSETTS OIL AND HAZARDOUS MATERIAL LIST TABLE OF CONTENTS TABLE 1 - MASSACHUSETTS OIL AND HAZARDOUS MATERIAL LIST (ALPHABETICAL LISTING) TABLE 2 - MASSACHUSETTS OIL AND HAZARDOUS MATERIAL LIST (BY CAS NUMBER ORDER) NOTES: The Massachusetts Oil and Hazardous Materials List (MOHML) contains oils and hazardous materials subject to 310 CMR 40.0000 and their reportable quantities (RQs) and reportable concentrations (RCs). These values are referred to in the notification requirements (310 CMR 40.0300). This list is provided both alphabetically in Table 1 and by Chemical Abstracts Service Number (CAS Number) in Table 2. The CAS number is a unique number assigned to a substance. Both tables identify other lists on which a substance appears by using name source codes. These codes are as follows: Name Source 1 - The Department of Transportation (DOT) Hazardous Materials List (49 CFR Part 172.101 Hazardous Materials Table) Name Source 2 - The Resource Conservation and Recovery Act Appendix VIII List (40 CFR Part 261 - Appendix VIII Hazardous Constituents) Name Source 3 - The Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) Hazardous Substance and Waste Stream Lists (40 CFR Part 302 - Table 302.4) Name Source 4 - The Extremely Hazardous Substance List as mandated by Superfund Amendments and Reauthorization Act, Title III, Section 302 (40 CFR Part 355 Appendices A and B) Name Source 5 - DEP Allowable Ambient Limits (AALs) and Drinking Water Guidelines Name Source 6 - The Massachusetts Substance List (MSL)(105 CMR 670.000: “Right to Know” Appendix A) Name Source 7 - The Chemical Abstracts name, 9th collective period, 1972-1976 Name Source 8 - The EPA Right to Know list, Section 313 of the Emergency Planning and Community Right to Know Act of 1986 (40 CFR Part 372.65). -

Agrilife Hazcom Program
TEXAS AGRILIFE RESEARCH AND TEXAS AGRILIFE EXTENSION HAZARD COMMUNICATION PROGRAM Revised August 2010 Table of Contents INTRODUCTION: ..................................................................................................................................... 3 PROGRAM EXEMPTIONS AND EXCEPTIONS ................................................................................. 3 RESEARCH LABORATORY EXEMPTIONS ....................................................................................... 4 DUTIES AND RESPONSIBILITIES ........................................................................................................ 4 THE SAFETY COORDINATOR .................................................................................................................. 4 THE CENTER DIRECTOR ......................................................................................................................... 4 THE SAFETY OFFICER ............................................................................................................................. 5 SUPERVISORS ........................................................................................................................................... 5 EMPLOYEES ............................................................................................................................................. 5 CONTRACTED CONSTRUCTION, REPAIR AND MAINTENANCE ............................................................. 5 HAZARDOUS CHEMICAL INVENTORY: .......................................................................................... -

Federal Register/Vol. 80, No. 33/Thursday, February 19, 2015
8956 Federal Register / Vol. 80, No. 33 / Thursday, February 19, 2015 / Proposed Rules ENVIRONMENTAL PROTECTION OW–2014–0797, by one of the following cannot read your comment due to AGENCY methods: technical difficulties and cannot contact • www.regulations.gov: Follow the you for clarification, EPA may not be 40 CFR Part 136 on-line instructions for submitting able to consider your comment. comments. Electronic files should avoid the use of [EPA–HQ–OW–2014–0797; FRL–9920–55– • Email: [email protected], special characters, any form of OW] Attention Docket ID number EPA–HQ– encryption, and be free of any defects or OW–2014–0797. viruses. RIN 2040–AF48 • Mail: Water Docket, Environmental Docket: All documents in the docket Protection Agency, Mail code: 4203M, Clean Water Act Methods Update Rule are listed in the www.regulations.gov 1200 Pennsylvania Ave. NW., for the Analysis of Effluent index. Although listed in the index, Washington, DC 20460. Attention some information in the docket is not Docket ID number EPA–HQ–OW–2014– AGENCY: Environmental Protection publicly available, e.g., CBI or other Agency (EPA). 0797. Please include a total of 3 copies. • Hand Delivery: Water Docket, EPA information whose disclosure is ACTION: Proposed rule. Docket Center, EPA West Building, restricted by statute. Certain other material, such as copyrighted material, SUMMARY: Room 3334, 1301 Constitution Ave. EPA proposes changes to will be publicly available only in hard pollutant analysis methods that are used NW., Washington, DC, Attention Docket ID number