WO 2011/156817 Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Use of Phosphate Solubilizing Bacteria to Leach Rare Earth Elements from Monazite-Bearing Ore
Minerals 2015, 5, 189-202; doi:10.3390/min5020189 OPEN ACCESS minerals ISSN 2075-163X www.mdpi.com/journal/minerals Article Use of Phosphate Solubilizing Bacteria to Leach Rare Earth Elements from Monazite-Bearing Ore Doyun Shin 1,2,*, Jiwoong Kim 1, Byung-su Kim 1,2, Jinki Jeong 1,2 and Jae-chun Lee 1,2 1 Mineral Resources Resource Division, Korea Institute of Geoscience and Mineral Resources (KIGAM), Gwahangno 124, Yuseong-gu, Daejeon 305-350, Korea; E-Mails: [email protected] (J.K.); [email protected] (B.K.); [email protected] (J.J.); [email protected] (J.L.) 2 Department of Resource Recycling Engineering, Korea University of Science and Technology, Gajeongno 217, Yuseong-gu, Daejeon 305-350, Korea * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +82-42-868-3616. Academic Editor: Anna H. Kaksonen Received: 8 January 2015 / Accepted: 27 March 2015 / Published: 2 April 2015 Abstract: In the present study, the feasibility to use phosphate solubilizing bacteria (PSB) to develop a biological leaching process of rare earth elements (REE) from monazite-bearing ore was determined. To predict the REE leaching capacity of bacteria, the phosphate solubilizing abilities of 10 species of PSB were determined by halo zone formation on Reyes minimal agar media supplemented with bromo cresol green together with a phosphate solubilization test in Reyes minimal liquid media as the screening studies. Calcium phosphate was used as a model mineral phosphate. Among the test PSB strains, Pseudomonas fluorescens, P. putida, P. rhizosphaerae, Mesorhizobium ciceri, Bacillus megaterium, and Acetobacter aceti formed halo zones, with the zone of A. -

COLUMBIUM - and RARE-EARTH ELEMENT-BEARING DEPOSITS at BOKAN MOUNTAIN, SOUTHEAST ALASKA by J
COLUMBIUM - AND RARE-EARTH ELEMENT-BEARING DEPOSITS AT BOKAN MOUNTAIN, SOUTHEAST ALASKA By J. Dean Warner and James C. Barker Alaska Field Operations Center * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * OFR - 33-89 UNITED STATES DEPARTMENT OF THE INTERIOR Manuel Lujan, Jr., Secretary BUREAU OF MINES T.S. Ary, Director TABLE OF CONTENTS Page Abstract ........................................................ 1 Introduction ....................... 2 Acknowledgments ................................................. 4 Location, access, and physiography .............................. 5 History and production ............. 5 Geologic setting .................................. 9 Trace element analyses of the peralkaline granite .............. 11 Nature of Bureau investigations ................................. 13 Sampling methods and analytical techniques .................... 13 Analytical interference, limitations, and self-shielding ...... 13 Resource estimation methods ................................... 15 Prospect evaluations .......................................... 16 Shear zones and fracture-controlled deposits .................... 18 Ross Adams mine ................. 18 Sunday Lake prospect.......................................... 21 I and L No. 1 and Wennie prospects ............................ 27 Other Occurrences ............................................. 31 Altered peralkaline granite ................................. 31 Dotson shear zone ........................................... 35 Resources .................................................. -

Specialty Direct Supply Drug List
DRAFT SPECIALTY DIRECT SUPPLY DRUG LIST (DSDL) 9/27/2005 Drug Description Effective Date 5-HT3 Receptor Antagonists ALOXI - SOLN 12/09/2004 ANZEMET - SOLN 12/09/2004 ANZEMET - TABS 12/09/2004 KYTRIL - SOLN 12/09/2004 KYTRIL - TABS 12/09/2004 Additional Solids ALPROSTADIL - POWD 12/09/2004 PROSTAGLANDIN E1 - POWD 12/09/2004 Agents for Gaucher Disease CEREZYME - SOLR 12/09/2004 Agents for Pheochromocytoma PHENTOLAMINE MESYLATE - SOLR 12/09/2004 REGITINE - SOLR 12/09/2004 Alkylating Agents CARBOPLATIN - SOLN 12/09/2004 CARBOPLATIN - SOLR 12/09/2004 CISPLATIN - SOLN 12/09/2004 CISPLATIN AQ - SOLN 12/09/2004 ELOXATIN - SOLR 12/09/2004 MYLERAN - TABS 12/09/2004 PARAPLATIN - SOLN 12/09/2004 PARAPLATIN - SOLR 12/09/2004 PLATINOL - SOLN 12/09/2004 PLATINOL AQ - SOLN 12/09/2004 THIOPLEX - SOLR 12/09/2004 THIOTEPA - SOLR 12/09/2004 Alpha-Proteinase Inhibitor (Human) ARALAST - SUSR 07/01/2005 PROLASTIN - SUSR 07/01/2005 ZEMAIRA - SOLR 07/01/2005 AMINOGLYCOSIDES APOGEN - SOLN 12/09/2004 GARAMYCIN - SOLN 12/09/2004 GENTAMICIN SULFATE - SOLN 12/09/2004 G-MYCIN - SOLN 12/09/2004 JENAMICIN - SOLN 12/09/2004 NEBCIN - SOLN 12/09/2004 NEBCIN MDV - SOLN 12/09/2004 STORZ-G - SOLN 12/09/2004 TOBI - NEBU 12/09/2004 TOBRAMYCIN SULFATE - SOLN 12/09/2004 TOBRAMYCIN SULFATE FLIPTO - SOLN 12/09/2004 Antianxiety Agents - Misc. rev A Page 1 of 13 MaineCare Direct Supply Drug List (DSDL) Drug Description Effective Date Antianxiety Agents - Misc. - Continued - DROPERIDOL - POWD 12/09/2004 DROPERIDOL - SOLN 12/09/2004 INAPSINE - SOLN 12/09/2004 Antiarrhythmics Type III -

(12) Patent Application Publication (10) Pub. No.: US 2014/0166788 A1 Pearse Et Al
US 2014O166788A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0166788 A1 Pearse et al. (43) Pub. Date: Jun. 19, 2014 (54) METHOD AND SYSTEM FOR MAGNETIC (52) U.S. Cl. SEPARATION OF RARE EARTHIS CPC. B03C I/02 (2013.01); B02C 23/08 (2013.01); B02C23/20 (2013.01) (76) Inventors: Gary Pearse, Ottawa (CA); Jonathan USPC .......... 241/20: 209/212: 209/214; 241/24.14; Borduas, Montreal (CA); Thomas 241/21 Gervais, Montreal (CA); David Menard, Laval (CA); Djamel Seddaoui, Repentigny (CA); Bora Ung, Quebec (CA) (57) ABSTRACT (21) Appl. No.: 13/822,363 A system and a method for separating rare earth element (22) PCT Filed: Aug. 15, 2012 compounds from a slurry of mixed rare earth element com pounds, comprising flowing the slurry of mixed rare earth (86). PCT No.: PCT/CA12/50.552 element compounds through at least a first channel rigged S371 (c)(1), with at least a first magnet along a length thereof and con (2), (4) Date: May 17, 2013 nected to at least a first output channel at the position of the Publication Classification magnet, and retrieving individual rare earth element com pounds and/or groups of rare earth element compounds, sepa (51) Int. Cl. rated from the slurry as they are selectively attracted by the BO3C I/02 (2006.01) magnet and directed in the corresponding output channel B2C 23/20 (2006.01) according to their respective ratio of magnetic Susceptibility B2C 23/08 (2006.01) (AX) to specific density (Ap). Ferromagnetic particles are dia?pararnegnetic particles Submitted to a magnetic torque remain relatively unaffected by the torque As a result they roll left \\ Carried right by the slow rotating drum Patent Application Publication Jun. -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -

Some Uses of Mischmetall in Organic Synthesis
REVIEW 9 Mischmetall in organic synthesis Organic synthesis Organic synthesis New from Acros Organics Marie-Isabelle Lannou, Florence Hélion and Jean-Louis Namy Laboratoire de Catalyse Moléculaire, associé au CNRS, ICMO, Bat 420, Université de Paris-Sud, 91405, Orsay, France Some uses of mischmetall in organic synthesis ∗ Marie-Isabelle Lannou, Florence Hélion and Jean-Louis Namy Laboratoire de Catalyse Moléculaire, associé au CNRS, ICMO, Bat 420, Université Paris-Sud, 91405, Orsay, France. Abstract: Mischmetall, an alloy of the light lanthanides, has been used in a variety of organic reactions, either as a coreductant in samarium(II)-mediated reactions (Barbier and Grignard-type reactions, pinacolic coupling reactions) or as the promoter of Reformatsky-type reactions. It has been also employed as the starting material for easy syntheses of lanthanide trihalides, the reactivity of which has been explored in Imamoto and Luche-Fukuzawa reactions and in Mukaiyama aldol reactions. Keywords: mischmetall, samarium, lanthanide, catalysis, organic reactions Introduction The use of rare earth compounds in organic chemistry has grown considerably during the past twenty years. Mainly samarium, cerium, lanthanum, ytterbium, neodymium, dysprosium, lutetium, scandium and yttrium metals and derivatives have been studied. These elements clearly differ in terms of reactivity. For instance, samarium(II) compounds can be used as powerful reductants whereas cerium(IV) ones are strong oxidants, some scandium and ytterbium(III) derivatives are very efficient catalysts in a variety of reactions and chemistry of lanthanum metal shows special features. We thought however that in many reactions, a mixture of lanthanide could be used instead of individual element without significant change in results. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Changes Highlighted Final Version Date of Issue: 23Rd December 2015
EPHMRA ANATOMICAL CLASSIFICATION GUIDELINES 2016 Section A Changed Classes/Guidelines: Changes Highlighted Final Version Date of issue: 23rd December 2015 1 A2B ANTIULCERANTS R1997r2 016 Combinations of specific antiulcerants with anti-infectives against Helicobacter pylori are classified according to the anti-ulcerant substance. For example, proton pump inhibitors in combination with these anti-infectives are classified in A2B2. A2B1 H2 antagonists R2002 Includes, for example, cimetidine, famotidine, nizatidine, ranitidine, roxatidine. Combinations of low dose H2 antagonists with antacids are classified with antacids in A2A6. A2B2 Acid Proton pump inhibitors R2003r2 016 Includes esomeprazole, lansoprazole, omeprazole, pantoprazole, rabeprazole. A2B3 Prostaglandin antiulcerants Includes misoprostol, enprostil. A2B4 Bismuth antiulcerants Includes combinations with antacids. A2B9 All other antiulcerants R2002r2 016 Includes all other products specifically stated to be antiulcerants even when containing antispasmodics (see A3). Combinations of low dose H2 antagonists with antacids are classified with antacids in A2A6. Included are, eg carbenoxolone, gefarnate, pirenzepine, proglumide, sucralfate and sofalcone. Herbal combinations are classified in A2C. In Japan, Korea and Taiwan only, sulpiride and other psycholeptics indicated for ulcer use are also included in this group, whilst in all other countries, these compounds are classified in N5A9. Products containing rebamipide for gastric mucosal protection are classified here. Products containing rebamipide and indicated for dry eye are classified in S1K9. A2C OTHER STOMACH DISORDER PREPARATIONS R1994 Includes herbal preparations and also plain alginic acid. Combinations of antacids with alginic acid are in A2A1. 2 A4 ANTIEMETICS AND ANTINAUSEANTS A4A ANTIEMETICS AND ANTINAUSEANTS R1996 Products indicated for vertigo and Meniere's disease are classified in N7C. Gastroprokinetics are classified in A3F. -
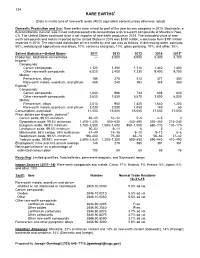
Mineral Commodity Summaries 2016
134 RARE EARTHS1 [Data in metric tons of rare-earth oxide (REO) equivalent content unless otherwise noted] Domestic Production and Use: Rare earths were mined for part of the year by one company in 2015. Bastnäsite, a fluorocarbonate mineral, was mined and processed into concentrates and rare-earth compounds at Mountain Pass, CA. The United States continued to be a net importer of rare-earth products in 2015. The estimated value of rare- earth compounds and metals imported by the United States in 2015 was $150 million, a decrease from $191 million imported in 2014. The estimated distribution of rare earths by end use was as follows, in decreasing order: catalysts, 60%; metallurgical applications and alloys, 10%; ceramics and glass, 10%; glass polishing, 10%; and other, 10%. Salient Statistics—United States: 2011 2012 2013 2014 2015e Production, bastnäsite concentrates — 3,000 5,500 5,400 4,100 Imports:2 Compounds: Cerium compounds 1,120 1,390 1,110 1,440 1,400 Other rare-earth compounds 6,020 3,400 7,330 9,400 9,700 Metals: Ferrocerium, alloys 186 276 313 371 360 Rare-earth metals, scandium, and yttrium 468 240 393 348 460 Exports:2 Compounds: Cerium compounds 1,640 996 734 608 600 Other rare-earth compounds 3,620 1,830 5,570 3,800 6,000 Metals: Ferrocerium, alloys 2,010 960 1,420 1,640 1,200 Rare-earth metals, scandium, and yttrium 3,030 2,080 1,050 140 60 Consumption, estimated 11,000 15,000 15,000 17,000 17,000 Price, dollars per kilogram, yearend:3 Cerium oxide, 99.5% minimum 40–45 10–12 5–6 4–5 2 Dysprosium oxide, 99.5% minimum 1,400–1,420 -

Lääkealan Turvallisuus- Ja Kehittämiskeskuksen Päätös
Lääkealan turvallisuus- ja kehittämiskeskuksen päätös N:o xxxx lääkeluettelosta Annettu Helsingissä xx päivänä maaliskuuta 2016 ————— Lääkealan turvallisuus- ja kehittämiskeskus on 10 päivänä huhtikuuta 1987 annetun lääke- lain (395/1987) 83 §:n nojalla päättänyt vahvistaa seuraavan lääkeluettelon: 1 § Lääkeaineet ovat valmisteessa suolamuodossa Luettelon tarkoitus teknisen käsiteltävyyden vuoksi. Lääkeaine ja sen suolamuoto ovat biologisesti samanarvoisia. Tämä päätös sisältää luettelon Suomessa lääk- Liitteen 1 A aineet ovat lääkeaineanalogeja ja keellisessä käytössä olevista aineista ja rohdoksis- prohormoneja. Kaikki liitteen 1 A aineet rinnaste- ta. Lääkeluettelo laaditaan ottaen huomioon lää- taan aina vaikutuksen perusteella ainoastaan lää- kelain 3 ja 5 §:n säännökset. kemääräyksellä toimitettaviin lääkkeisiin. Lääkkeellä tarkoitetaan valmistetta tai ainetta, jonka tarkoituksena on sisäisesti tai ulkoisesti 2 § käytettynä parantaa, lievittää tai ehkäistä sairautta Lääkkeitä ovat tai sen oireita ihmisessä tai eläimessä. Lääkkeeksi 1) tämän päätöksen liitteessä 1 luetellut aineet, katsotaan myös sisäisesti tai ulkoisesti käytettävä niiden suolat ja esterit; aine tai aineiden yhdistelmä, jota voidaan käyttää 2) rikoslain 44 luvun 16 §:n 1 momentissa tar- ihmisen tai eläimen elintoimintojen palauttami- koitetuista dopingaineista annetussa valtioneuvos- seksi, korjaamiseksi tai muuttamiseksi farmako- ton asetuksessa kulloinkin luetellut dopingaineet; logisen, immunologisen tai metabolisen vaikutuk- ja sen avulla taikka terveydentilan -

Anatomical Classification Guidelines V2021 EPHMRA ANATOMICAL CLASSIFICATION GUIDELINES 2021
EPHMRA ANATOMICAL CLASSIFICATION GUIDELINES 2021 Anatomical Classification Guidelines V2021 "The Anatomical Classification of Pharmaceutical Products has been developed and maintained by the European Pharmaceutical Marketing Research Association (EphMRA) and is therefore the intellectual property of this Association. EphMRA's Classification Committee prepares the guidelines for this classification system and takes care for new entries, changes and improvements in consultation with the product's manufacturer. The contents of the Anatomical Classification of Pharmaceutical Products remain the copyright to EphMRA. Permission for use need not be sought and no fee is required. We would appreciate, however, the acknowledgement of EphMRA Copyright in publications etc. Users of this classification system should keep in mind that Pharmaceutical markets can be segmented according to numerous criteria." © EphMRA 2021 Anatomical Classification Guidelines V2021 CONTENTS PAGE INTRODUCTION A ALIMENTARY TRACT AND METABOLISM 1 B BLOOD AND BLOOD FORMING ORGANS 28 C CARDIOVASCULAR SYSTEM 36 D DERMATOLOGICALS 51 G GENITO-URINARY SYSTEM AND SEX HORMONES 58 H SYSTEMIC HORMONAL PREPARATIONS (EXCLUDING SEX HORMONES) 68 J GENERAL ANTI-INFECTIVES SYSTEMIC 72 K HOSPITAL SOLUTIONS 88 L ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS 96 M MUSCULO-SKELETAL SYSTEM 106 N NERVOUS SYSTEM 111 P PARASITOLOGY 122 R RESPIRATORY SYSTEM 124 S SENSORY ORGANS 136 T DIAGNOSTIC AGENTS 143 V VARIOUS 145 Anatomical Classification Guidelines V2021 INTRODUCTION The Anatomical Classification was initiated in 1971 by EphMRA. It has been developed jointly by Intellus/PBIRG and EphMRA. It is a subjective method of grouping certain pharmaceutical products and does not represent any particular market, as would be the case with any other classification system. -

Bastnaesite Beneficiation by Froth Flotation and Gravity Separation
BASTNAESITE BENEFICIATION BY FROTH FLOTATION AND GRAVITY SEPARATION by Nathanael Williams A thesis submitted to the Faculty and the Board of Trustees of the Colorado School of Mines in partial fulfillment of the requirements for the degree of Master of Science (Metallurgical and Materials Engineering). Golden, Colorado Date___________ Signed:_______________________ Nathanael Williams Date___________ Signed:_______________________ Dr. Corby Anderson Thesis Advisor Date:___________ Signed:_______________________ Dr. Angus Rockett Department Head Department of Metallurgical and Materials Engineering ii ABSTRACT Rare earth elements are in high demand in the United States. Independence from the importation of rare earths is essential to alleviate dependence on China for these rare earth elements. Bastnaesite, a rare earth fluorocarbonate, is one of the most abundant sources of rare earths in the United States. It is a fluorocarbonate mineral containing primarily cerium and lanthanum. The largest rare earth mine in the United States is Mountain Pass. This research was done to find a way to combine flotation with novel collectors and gravity separation techniques to reach an enhanced grade and recovery of rare earth elements while rejecting the gangue minerals, calcite, barite and silicate minerals. The main economic driving force is the price of hydrochloric acid in downstream processes, as calcite is an acid consumer. Surface chemistry analysis was completed using adsorption density, zeta potential, and microflotation on both gravity concentrates and run of mine ore samples. Four collectors were examined. These were N,2.dihydroxybenzamide, N-hydroxycyclohexanecarboxamide, N,3. dihydroxy-2.naphthamide, and N-hydroxyoleamide. Through this analysis it was determined that, to obtain the desired results, that flotation would be the rougher stage and the gravity separation would be utilized as the cleaner stage.