Molybdenum, Ferromolybdenum, and Ammonium Molybdate, Phosphoric
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Use of Phosphate Solubilizing Bacteria to Leach Rare Earth Elements from Monazite-Bearing Ore
Minerals 2015, 5, 189-202; doi:10.3390/min5020189 OPEN ACCESS minerals ISSN 2075-163X www.mdpi.com/journal/minerals Article Use of Phosphate Solubilizing Bacteria to Leach Rare Earth Elements from Monazite-Bearing Ore Doyun Shin 1,2,*, Jiwoong Kim 1, Byung-su Kim 1,2, Jinki Jeong 1,2 and Jae-chun Lee 1,2 1 Mineral Resources Resource Division, Korea Institute of Geoscience and Mineral Resources (KIGAM), Gwahangno 124, Yuseong-gu, Daejeon 305-350, Korea; E-Mails: [email protected] (J.K.); [email protected] (B.K.); [email protected] (J.J.); [email protected] (J.L.) 2 Department of Resource Recycling Engineering, Korea University of Science and Technology, Gajeongno 217, Yuseong-gu, Daejeon 305-350, Korea * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +82-42-868-3616. Academic Editor: Anna H. Kaksonen Received: 8 January 2015 / Accepted: 27 March 2015 / Published: 2 April 2015 Abstract: In the present study, the feasibility to use phosphate solubilizing bacteria (PSB) to develop a biological leaching process of rare earth elements (REE) from monazite-bearing ore was determined. To predict the REE leaching capacity of bacteria, the phosphate solubilizing abilities of 10 species of PSB were determined by halo zone formation on Reyes minimal agar media supplemented with bromo cresol green together with a phosphate solubilization test in Reyes minimal liquid media as the screening studies. Calcium phosphate was used as a model mineral phosphate. Among the test PSB strains, Pseudomonas fluorescens, P. putida, P. rhizosphaerae, Mesorhizobium ciceri, Bacillus megaterium, and Acetobacter aceti formed halo zones, with the zone of A. -

COLUMBIUM - and RARE-EARTH ELEMENT-BEARING DEPOSITS at BOKAN MOUNTAIN, SOUTHEAST ALASKA by J
COLUMBIUM - AND RARE-EARTH ELEMENT-BEARING DEPOSITS AT BOKAN MOUNTAIN, SOUTHEAST ALASKA By J. Dean Warner and James C. Barker Alaska Field Operations Center * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * OFR - 33-89 UNITED STATES DEPARTMENT OF THE INTERIOR Manuel Lujan, Jr., Secretary BUREAU OF MINES T.S. Ary, Director TABLE OF CONTENTS Page Abstract ........................................................ 1 Introduction ....................... 2 Acknowledgments ................................................. 4 Location, access, and physiography .............................. 5 History and production ............. 5 Geologic setting .................................. 9 Trace element analyses of the peralkaline granite .............. 11 Nature of Bureau investigations ................................. 13 Sampling methods and analytical techniques .................... 13 Analytical interference, limitations, and self-shielding ...... 13 Resource estimation methods ................................... 15 Prospect evaluations .......................................... 16 Shear zones and fracture-controlled deposits .................... 18 Ross Adams mine ................. 18 Sunday Lake prospect.......................................... 21 I and L No. 1 and Wennie prospects ............................ 27 Other Occurrences ............................................. 31 Altered peralkaline granite ................................. 31 Dotson shear zone ........................................... 35 Resources .................................................. -

Molybdates: an Alternative to Hexavalent Chromates in Corrosion
Molybdates: An Alternative to Hexavalent Chromates 3ytzt7n2 in Corrosion Prevention and Control qz ,+ B3 F Stiles C. Thompson Sverdrup Technology, Inc. 626 Anchors St. NW Fort Walton Beach, FL 32548 (904) 833-7600 Introduction Hexavalent chromates have been the preferred corrosion inhibitor in military systems for years. Chromate conversion coatings are specified in MIL-STD-808, “Finishes, Materials and Processes for Corrosion Prevention and Control in Support Equipment ”, for aluminum, magnesium, and plated steel. A number of primers are formulated with zinc or strontium chromate. Passivation treatments for stainless steels frequently specify chromic acid. Some anodic coatings specify baths containing chromic and sulfuric acid and a pcst treatment or rinse with aqueous sodium dichromate. Even water-based primers formulated for volatile organic I compound (VOC) compliance in such areas as southern California contain chromate-based corrosion inhibitors. Chromates are decreasing in industrial use, however, as concerns about their environmental risks and toxicity increase. One pollution prevention approach is to substitute analogs of chromates in similar applications. Molybdate is one such analog of lower toxicity. Federal Environmental Regulations A number of environmental regulations now control hexavalent chromate. Toxic Substances Control Act (TSCA). Under TSCA, the Environmental Protection Agency @PA) must be notified 90 days prior to manufacture. EPA may restrict or prohibit manufacture where there is unreasonable risk to the environment. EPA also has the authority to require testing for health or environmental effects. No current regulation specifies molybdate. Resource Conservation and Recovery Act (RCRA). RCRA regulates the treatment, storage, transportation, and disposal of hazardous waste (HW). The EPA HW number for hexavalent chromium is D007. -

(12) Patent Application Publication (10) Pub. No.: US 2014/0166788 A1 Pearse Et Al
US 2014O166788A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0166788 A1 Pearse et al. (43) Pub. Date: Jun. 19, 2014 (54) METHOD AND SYSTEM FOR MAGNETIC (52) U.S. Cl. SEPARATION OF RARE EARTHIS CPC. B03C I/02 (2013.01); B02C 23/08 (2013.01); B02C23/20 (2013.01) (76) Inventors: Gary Pearse, Ottawa (CA); Jonathan USPC .......... 241/20: 209/212: 209/214; 241/24.14; Borduas, Montreal (CA); Thomas 241/21 Gervais, Montreal (CA); David Menard, Laval (CA); Djamel Seddaoui, Repentigny (CA); Bora Ung, Quebec (CA) (57) ABSTRACT (21) Appl. No.: 13/822,363 A system and a method for separating rare earth element (22) PCT Filed: Aug. 15, 2012 compounds from a slurry of mixed rare earth element com pounds, comprising flowing the slurry of mixed rare earth (86). PCT No.: PCT/CA12/50.552 element compounds through at least a first channel rigged S371 (c)(1), with at least a first magnet along a length thereof and con (2), (4) Date: May 17, 2013 nected to at least a first output channel at the position of the Publication Classification magnet, and retrieving individual rare earth element com pounds and/or groups of rare earth element compounds, sepa (51) Int. Cl. rated from the slurry as they are selectively attracted by the BO3C I/02 (2006.01) magnet and directed in the corresponding output channel B2C 23/20 (2006.01) according to their respective ratio of magnetic Susceptibility B2C 23/08 (2006.01) (AX) to specific density (Ap). Ferromagnetic particles are dia?pararnegnetic particles Submitted to a magnetic torque remain relatively unaffected by the torque As a result they roll left \\ Carried right by the slow rotating drum Patent Application Publication Jun. -

A Titrimetric Determination of Thorium John J
Ames Laboratory ISC Technical Reports Ames Laboratory 6-1954 A titrimetric determination of thorium John J. Ford Iowa State College James S. Fritz Iowa State College Follow this and additional works at: http://lib.dr.iastate.edu/ameslab_iscreports Part of the Chemistry Commons Recommended Citation Ford, John J. and Fritz, James S., "A titrimetric determination of thorium" (1954). Ames Laboratory ISC Technical Reports. 92. http://lib.dr.iastate.edu/ameslab_iscreports/92 This Report is brought to you for free and open access by the Ames Laboratory at Iowa State University Digital Repository. It has been accepted for inclusion in Ames Laboratory ISC Technical Reports by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. A titrimetric determination of thorium Abstract A rapid, accurate method for thorium is proposed in which thorium is titrated with a standard solution of ethylenediaminetetraacetic acid (EDTA). Alizarin Red S serves as the indicator, a sharp change from pink to yellow marking the end point of the titration. The method is selective for thorium although several cations and anions interfere. A preliminary extraction of thorium nitrate by mentyl oxide provides an excellent separation of thorium from most interfering ions. Keywords Ames Laboratory Disciplines Chemistry This report is available at Iowa State University Digital Repository: http://lib.dr.iastate.edu/ameslab_iscreports/92 Physical 'cieuccs Re· dmg Room UNITED STATES ATOMIC ENERGY COMMISSION ISC-520 A TITRIMETRIC DETERMINATION OF THORIUM By John J. Ford James S. Fritz June 1954 Technical Information Service, Oak Ridge, Tennessee J Subject Category, CHEMISTRY. Work performed under Contract No. -

WO 2011/156817 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date Χ t it o n 1 15 December 2011 (15.12.2011) WO 2011/156817 Al (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C02F1/58 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, (21) International Application Number: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, PCT/US20 11/040214 DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, 13 June 201 1 (13.06.201 1) KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (25) Filing Language: English NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (26) Publication Language: English SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 61/354,031 11 June 2010 ( 11.06.2010) (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant (for all designated States except US): MOLY- GM, KE, LR, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, CORP MINERALS LLC [US/US]; 561 Denver Tech ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, Center Pkwy, Suite 1000, Greenwood Village, CO 801 11 TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, (US). -

Some Uses of Mischmetall in Organic Synthesis
REVIEW 9 Mischmetall in organic synthesis Organic synthesis Organic synthesis New from Acros Organics Marie-Isabelle Lannou, Florence Hélion and Jean-Louis Namy Laboratoire de Catalyse Moléculaire, associé au CNRS, ICMO, Bat 420, Université de Paris-Sud, 91405, Orsay, France Some uses of mischmetall in organic synthesis ∗ Marie-Isabelle Lannou, Florence Hélion and Jean-Louis Namy Laboratoire de Catalyse Moléculaire, associé au CNRS, ICMO, Bat 420, Université Paris-Sud, 91405, Orsay, France. Abstract: Mischmetall, an alloy of the light lanthanides, has been used in a variety of organic reactions, either as a coreductant in samarium(II)-mediated reactions (Barbier and Grignard-type reactions, pinacolic coupling reactions) or as the promoter of Reformatsky-type reactions. It has been also employed as the starting material for easy syntheses of lanthanide trihalides, the reactivity of which has been explored in Imamoto and Luche-Fukuzawa reactions and in Mukaiyama aldol reactions. Keywords: mischmetall, samarium, lanthanide, catalysis, organic reactions Introduction The use of rare earth compounds in organic chemistry has grown considerably during the past twenty years. Mainly samarium, cerium, lanthanum, ytterbium, neodymium, dysprosium, lutetium, scandium and yttrium metals and derivatives have been studied. These elements clearly differ in terms of reactivity. For instance, samarium(II) compounds can be used as powerful reductants whereas cerium(IV) ones are strong oxidants, some scandium and ytterbium(III) derivatives are very efficient catalysts in a variety of reactions and chemistry of lanthanum metal shows special features. We thought however that in many reactions, a mixture of lanthanide could be used instead of individual element without significant change in results. -
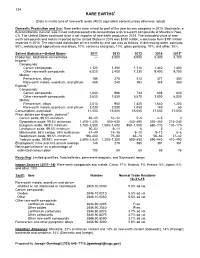
Mineral Commodity Summaries 2016
134 RARE EARTHS1 [Data in metric tons of rare-earth oxide (REO) equivalent content unless otherwise noted] Domestic Production and Use: Rare earths were mined for part of the year by one company in 2015. Bastnäsite, a fluorocarbonate mineral, was mined and processed into concentrates and rare-earth compounds at Mountain Pass, CA. The United States continued to be a net importer of rare-earth products in 2015. The estimated value of rare- earth compounds and metals imported by the United States in 2015 was $150 million, a decrease from $191 million imported in 2014. The estimated distribution of rare earths by end use was as follows, in decreasing order: catalysts, 60%; metallurgical applications and alloys, 10%; ceramics and glass, 10%; glass polishing, 10%; and other, 10%. Salient Statistics—United States: 2011 2012 2013 2014 2015e Production, bastnäsite concentrates — 3,000 5,500 5,400 4,100 Imports:2 Compounds: Cerium compounds 1,120 1,390 1,110 1,440 1,400 Other rare-earth compounds 6,020 3,400 7,330 9,400 9,700 Metals: Ferrocerium, alloys 186 276 313 371 360 Rare-earth metals, scandium, and yttrium 468 240 393 348 460 Exports:2 Compounds: Cerium compounds 1,640 996 734 608 600 Other rare-earth compounds 3,620 1,830 5,570 3,800 6,000 Metals: Ferrocerium, alloys 2,010 960 1,420 1,640 1,200 Rare-earth metals, scandium, and yttrium 3,030 2,080 1,050 140 60 Consumption, estimated 11,000 15,000 15,000 17,000 17,000 Price, dollars per kilogram, yearend:3 Cerium oxide, 99.5% minimum 40–45 10–12 5–6 4–5 2 Dysprosium oxide, 99.5% minimum 1,400–1,420 -

FALL2015.Pdf
Introducing… In Meso In Situ Serial Crystallography mitegen.com/imisx Easy Crystallizaon in LCP • Simple in situ X-ray Diffracon • Standard Goniometer Compable • Serial Crystallography Enabling • Advanced Dual-Sandwich Design Rethink what your LCP crystallizaon plate is capable of. EZ-Cut LCP LCP Tools Easy Access LCP Crystallizaon Plate Starter Kits, Syringes, Lipids & More Take your crystallizaon in LCP to the next level with MiTeGen’s new LCP line mitegen.com/lcp ACA - Structure Matters www.AmerCrystalAssn.org Table of Contents 2 President’s Column 2-4 News from Canada 4 From the Editor's Desk 5-6 Update on Structural Dynamics Chris Cahill 7 What's on the Cover ACA President 8-11 Poster Prizes in Philadelphia 12 Undergraduate Student Reception in Philadelphia Contributors to this Issue What's on the Cover 13-51 Philadelphia ACA Meeting Page 7 21 Index of Advertisers 53-54 2015 ACA Summer Course 56 What's New on the ACA History Website Spotlight on Stamps Juanma García-Ruiz Net RefleXions 15 Plenary Lecturer in Philadelphia 58-59 Puzzle Corner 59 YSSIG Activities 60-61 48th Erice International School of Crystallography 61-62 Updates from 2014-2015 AIP Science & Technology Fellows 62 CSD50 Symposium Daniel Rabinovich 64-65 News & Awards Spotlight on Stamps 65-66 Book Reviews 66-67 2015 Wisconsin Crystal-Growing Competition 68 Net RefleXions 69 CCDC Data Deposition Service Enhancement 70-71 ACA 2016 Denver Preview 72 Future Meetings Anastasiya Vinokur Net RefleXions Contributions to ACA RefleXions may be sent to either of the Editors: Please address matters pertaining to advertisements, membership Judith L. -

Bastnaesite Beneficiation by Froth Flotation and Gravity Separation
BASTNAESITE BENEFICIATION BY FROTH FLOTATION AND GRAVITY SEPARATION by Nathanael Williams A thesis submitted to the Faculty and the Board of Trustees of the Colorado School of Mines in partial fulfillment of the requirements for the degree of Master of Science (Metallurgical and Materials Engineering). Golden, Colorado Date___________ Signed:_______________________ Nathanael Williams Date___________ Signed:_______________________ Dr. Corby Anderson Thesis Advisor Date:___________ Signed:_______________________ Dr. Angus Rockett Department Head Department of Metallurgical and Materials Engineering ii ABSTRACT Rare earth elements are in high demand in the United States. Independence from the importation of rare earths is essential to alleviate dependence on China for these rare earth elements. Bastnaesite, a rare earth fluorocarbonate, is one of the most abundant sources of rare earths in the United States. It is a fluorocarbonate mineral containing primarily cerium and lanthanum. The largest rare earth mine in the United States is Mountain Pass. This research was done to find a way to combine flotation with novel collectors and gravity separation techniques to reach an enhanced grade and recovery of rare earth elements while rejecting the gangue minerals, calcite, barite and silicate minerals. The main economic driving force is the price of hydrochloric acid in downstream processes, as calcite is an acid consumer. Surface chemistry analysis was completed using adsorption density, zeta potential, and microflotation on both gravity concentrates and run of mine ore samples. Four collectors were examined. These were N,2.dihydroxybenzamide, N-hydroxycyclohexanecarboxamide, N,3. dihydroxy-2.naphthamide, and N-hydroxyoleamide. Through this analysis it was determined that, to obtain the desired results, that flotation would be the rougher stage and the gravity separation would be utilized as the cleaner stage. -

Tungsten Removal from Molybdate Solutions Using Chelating Ion-Exchange Resin: Equilibrium Adsorption Isotherm and Kinetics
J. Cent. South Univ. (2016) 23: 1052−1057 DOI: 10.1007/s11771-016-0354-y Tungsten removal from molybdate solutions using chelating ion-exchange resin: Equilibrium adsorption isotherm and kinetics ZHU Xian-zheng(朱先正), HUO Guang-sheng(霍广生), NI Jie(倪捷), SONG Qiong(宋琼) School of Metallurgy and Environment, Central South University, Changsha 410083, China © Central South University Press and Springer-Verlag Berlin Heidelberg 2016 Abstract: The equilibrium adsorption isotherm and kinetic of the sorption process for W and Mo on macro chelating resin D403 were investigated on single Na2MoO4 and Na2WO4 solutions. The sorption isotherm results show that the adsorption process of W obeys the Freundlich model very well whereas the exchange process with Mo approximately follows the Henry model. The kinetic experiments show that the intraparticle diffusion process was the rate-determining step for W sorption on the resin, and the corresponding activation energy is calculated to be 21.976 kJ/mol. Key words: tungsten; molybdate; ion-exchange resin; isotherms; kinetics tin (IV) oxide hydrate [14], that would preferentially 1 Introduction form precipitations with W under certain conditions. The separation was effective but a large number of The separation between W and Mo has been a precipitation agents were required and the impurity ions national difficulty due to their extremely similar accompanied with the added reagents were inevitable, physicochemical properties, resulting from the increasing the impurity-removal burden. lanthanide contraction [1]. Additionally, according to the Inspired by the different affinities towards O industrial requirement, WO3 impurity in final ammonium between W and Mo, we discovered a novel resin (D403) molybdate product should be restricted to below containing meglumine group which exhibited marvelous 150×10−6 (mass fraction) [2], making W removal from separation effects between Mo and W at pH above 8.0, at molybdate solutions particularly important. -

A High-Affinity Molybdate Transporter in Eukaryotes
A high-affinity molybdate transporter in eukaryotes Manuel Tejada-Jime´ nez, Angel´ Llamas, Emanuel Sanz-Luque, Aurora Galva´ n, and Emilio Ferna´ ndez* Departamento de Bioquı´micay Biologı´aMolecular, Facultad de Ciencias, Universidad de Co´rdoba, Campus de Rabanales, Edif. Severo Ochoa, 14071 Co´rdoba, Spain Edited by Bob B. Buchanan, University of California, Berkeley, CA, and approved October 29, 2007 (received for review May 17, 2007) Molybdenum is an essential element for almost all living beings, In contrast to bacteria, molybdate transporters in eukaryotes which, in the form of a molybdopterin-cofactor, participates in the are unknown, and genes similar to the bacterial molybdate active site of enzymes involved in key reactions of carbon, nitrogen, channel modB have not been found in sequenced eukaryotic and sulfur metabolism. This metal is taken up by cells in form of the genomes. Physiological data from the green alga Chlamydomo- oxyanion molybdate. Bacteria acquire molybdate by an ATP-binding- nas reinhardtii suggest the presence of at least two molybdate cassette (ABC) transport system in a widely studied process, but how transport systems that are related to the unlinked genetic loci Ni5 eukaryotic cells take up molybdenum is unknown because molybdate and Nit6 (10). Mutants defective at one of these loci are transporters have not been identified so far. Here, we report a phenotypically wild type but have a reduced molybdate transport eukaryotic high-affinity molybdate transporter, encoded by the activity; double mutants at both loci lack Moco and, thus, activity green alga Chlamydomonas reinhardtii gene MoT1. An antisense RNA of the molybdoenzyme nitrate reductase (11).