Download Vol. 6, No. 3
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pilgrimage Schedule
43RD ANNUAL SPRING WILDFLOWER PILGRIMAGE MAY 04 - 06, 2012 CLAYTON, RABUN COUNTY, GEORGIA & ADJACENT AREAS Clayton, our headquarters for the 43rd Spring Wildflower Pilgrimage, is nestled in the Blue Ridge Mountains of northeast Georgia just a stone’s throw from the Carolinas. The Sumter National Forest is to the east, the Nantahala National Forest is to the north, and the Chattahoochee National Forest is all around Clayton. Some of Georgia’s highest mountains are but a short drive. A diverse group of interested persons from four or more states are expected to participate. The pilgrimage will consist of a Friday night social with a program and great food, a Saturday banquet with a special presentation program and more great food, and fantastic field trips to some very special places located in the region. Clayton and the surrounding area have a diversity of interesting shops that will entice you to shop for local arts, crafts, and foods. Clayton is the county seat for Rabun County, founded in 1819 from land formally inhabited by the Cherokee and named for Governor William Rabun. The 377 square miles of Rabun County comprise the most northeastern section of Georgia’s Blue Ridge Geographical Province, a region that encompasses a mere 5% of Georgia. Sixty percent of the county is public lands under the management of the US Forest Service or the Georgia Department of Natural Resources. This beautiful area of scenic valleys, high rugged mountains, clear streams, and lush forests is attractive year round, but offers a special floristic bounty each spring. Join the Georgia Botanical Society for the Annual Spring Wildflower Pilgrimage and share in this rich and beautiful bounty. -

Hiking 34 Mountain Biking 37 Bird Watching 38 Hunting 38 Horseback Riding 38 Rock Climbing 40 Gliding 40 Watersports 41 Shopping 44 Antiquing 45 Craft Hunting 45
dventure Guide to the Great Smoky Mountains 2nd Edition Blair Howard HUNTER HUNTER PUBLISHING, INC. 130 Campus Drive Edison, NJ 08818-7816 % 732-225-1900 / 800-255-0343 / fax 732-417-1744 Web site: www.hunterpublishing.com E-mail: [email protected] IN CANADA: Ulysses Travel Publications 4176 Saint-Denis, Montréal, Québec Canada H2W 2M5 % 514-843-9882 ext. 2232 / fax 514-843-9448 IN THE UNITED KINGDOM: Windsor Books International The Boundary, Wheatley Road, Garsington Oxford, OX44 9EJ England % 01865-361122 / fax 01865-361133 ISBN 1-55650-905-7 © 2001 Blair Howard All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form, or by any means, elec- tronic, mechanical, photocopying, recording, or otherwise, without the written permission of the publisher. This guide focuses on recreational activities. As all such activities contain elements of risk, the publisher, author, affiliated individuals and compa- nies disclaim any responsibility for any injury, harm, or illness that may occur to anyone through, or by use of, the information in this book. Every effort was made to insure the accuracy of information in this book, but the publisher and author do not assume, and hereby disclaim, any liability or any loss or damage caused by errors, omissions, misleading information or potential travel problems caused by this guide, even if such errors or omis- sions result from negligence, accident or any other cause. Cover photo by Michael H. Francis Maps by Kim André, © 2001 Hunter -

Biological Evaluation
United States Department of Agriculture Forest Service March 2018 Biological Evaluation Prospect Hamby Project Tusquitee Ranger District, Nantahala National Forest Cherokee County, North Carolina For Additional Information Contact: Tusquitee Ranger District 123 Woodland Drive Murphy, North Carolina 28906 (828) 837-5152 2-1 Table of Contents 1.0 INTRODUCTION .......................................................................................................................... 2 1.1 Proposed Action ......................................................................................................................... 2 1.2 Species Considered ..................................................................................................................... 2 2.0 PROPOSED, ENDANGERED, and THREATENED SPECIES ................................................... 3 2.1 Aquatic Resources ...................................................................................................................... 3 2.2 Botanical Resources ................................................................................................................... 6 2.3 Wildlife Resources ..................................................................................................................... 8 2.4 Effects Determinations for Proposed, Endangered, and Threatened Species ........................... 14 3.0 SENSITIVE SPECIES ................................................................................................................. 14 3.1 Aquatic -

Public Law 88-577, September 3, 1964) and Related Acts, the U.S
DEPARTMENT OF THE INTERIOR MISCELLANEOUS FIELD STUDIES UNITED STATES GEOLOGICAL SURVEY MAP MF-1587-C PAMPHLET MINERAL RESOURCE POTENTIAL OF THE SNOWBIRD ROADLESS AREA GRAHAM COUNTY, NORTH CAROLINA By Frank G. Lesure, U.S. Geological Survey and Mark L. Chatman, U.S. Bureau of Mines 1983 Studies Related to Wilderness Under the provisions of the Wilderness Act (Public Law 88-577, September 3, 1964) and related acts, the U.S. Geological Survey and the U.S. Bureau of Mines have been conducting mineral surveys of wilderness and primitive areas. Areas officially designated as "wilderness," "wild," or "canoe" when the act was passed were incorporated into the National Wilderness Preservation System, and some of them are presently being studied. The act provided that areas under consideration for wilderness designation should be studied for suitability for incorporation into the Wilderness System. The mineral surveys constitute one aspect of the suitability studies. The act directs that the results of such surveys are to be made available to the public and be submitted to the President and the Congress. This report discusses the results of a mineral survey of the Snowbird Roadless Area (08-061), Nantahala National Forest, Graham County, North Carolina. The area was classified as a further planning area during the Second Roadless Area Review and Evaluation (RARE II) by the U.S. Forest Service, January 1979. MINERAL RESOURCE POTENTIAL SUMMARY STATEMENT The Snowbird Roadless Area includes 8490 acres of rugged wooded terrain in the Nantahala National Forest, Graham County, N. C. The area is underlain by folded metasedimentary rocks of the Great Smoky Group of Late Proterozoic age, and has a low potential for mineral resources. -
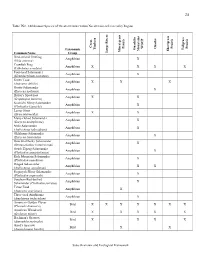
State Overview and Ecological Framework Table IN2. Oklahoma's
24 Table IN2. Oklahoma's Species of Greatest Conservation Need Cross-referenced by Region grass - Cross Prairie Prairie Prairie WGCP Ozarks Timbers Tallgrass Taxonomic Ouachita Mountains Shortgrass Mixed Common Name Group Large Rivers Bird-voiced Treefrog Amphibian X (Hyla avivoca) Crawfish Frog Amphibian X X X X (Lithobates areolata) Four-toed Salamander Amphibian X (Hemidactylium scutatum) Green Toad Amphibian X X X (Anaxyrus debilis) Grotto Salamander Amphibian X (Eurycea spelaeus) Hurter's Spadefoot Amphibian X X (Scaphiopus hurterii) Kiamichi Slimy Salamander Amphibian X (Plethodon kiamichi) Lesser Siren Amphibian X X (Siren intermedia) Many-ribbed Salamander Amphibian X (Eurycea multiplicata) Mole Salamander Amphibian X (Ambystoma talpoideum) Oklahoma Salamander Amphibian X (Eurycea tynerensis) Ouachita Dusky Salamander Amphibian X (Desmognathus brimleyorum) Ozark Zigzag Salamander Amphibian X (Plethodon angusticlavius) Rich Mountain Salamander Amphibian X (Plethodon ouachitae) Ringed Salamander Amphibian X X (Ambystoma annulatum) Sequoyah Slimy Salamander Amphibian X (Plethodon sequoyah) Southern Red-backed Amphibian X Salamander (Plethodon serratus) Texas Toad Amphibian X (Anaxyrus speciosus) Three-toed Amphiuma Amphibian X (Amphiuma tridactylum) American Golden Plover Bird X X X X X X X (Pluvialis dominica) American Woodcock Bird X X X X X (Scolopax minor) Bachman's Sparrow Bird X X X X (Aimophila aestivalis) Baird's Sparrow Bird X X (Ammodramus bairdii) State Overview and Ecological Framework 25 grass - ntains Cross Large Rivers -

Florida MSTA Newsletter for May 2020
The Florida Gator Tale Newsletter of the Florida Chapter of the Motorcycle Sport Touring Association June 2021 - Volume 14 Issue 6 In the June 2021 Issue: Feature Article Page 1 – Feature Article Page 9 – Safety Talk Page 11 – Florida News Page 14 – Florida Rides STAR 2021 Route Guide Page 15 – Future Florida Rides MSTA Page 16 – Florida MSTA Apparel Page 16 – Classified Ads Contact Information: South Florida Director: Van VanSteelant – [email protected] Central Florida Director: Carl Swofford – [email protected] Florida Gator Tale Editor: Kim Longacre – [email protected] For the upcoming STAR 2021 at the Canaan Valley Resort in Davis, West Virginia (June 13-16, 2021), the MSTA offers an online STAR 2021 Route Guide at http://star21.flybyweek.com/RouteGuide.html. This year, in addition to the GPX navigation files, each route includes a link to an online map image and a pdf document of the map and Garmin route that you can download. Super easy!! Here are summary descriptions of each of the 22 STAR routes in the guide: 01 River RunsThru It RouteType: TOURING BIKE-FRIENDLY Navigation difficulty: AVERAGE Distance: 196 miles. Lunch Stop: Apple Annie's near Morgantown, WV Direction from Canaan Valley: NW, N, NE, CLOCKWISE Comments: Something for big bikes, 2-up with some 1.5 lane twisty pavement to work up appetites. This route leans more towards civilization. 02 Smoke Hole to the Knob RouteType: TOURING BIKE-FRIENDLY, DESTINATION- Spruce Knob, CURVY route. Navigation difficulty: AVERAGE Distance: 179 miles. Lunch Stop: Hollow Restaurant in Franklin, WV, and Ice Cream at Moe Fatz if desired. -

2017 Annual Report
Annual Report 2017 Seven land protection projects Completed in one year Many things made 2017 our biggest year to date: Great project partners, a staff and board that are ardently dedicated to conserving West Virginia’s special places, generous supporters, lots of patience, and WVLT’s availability to respond to multiple requests for assistance… which continue to increase with each month. These lands are now permanently protected. Yes, permanently. As in…. forever. Signed, sealed, and recorded in deed books in their respective counties. A public park for climbing, family histories, a waterfall, wildlife habitat, hiking trails, beautiful forests and streams, agricultural land: All great places that will keep West Virginia wild and wonderful. Good projects often take a long time to complete. One of 2017’s successes was in the works for five years! With conservation easements, we take our time to make sure that landowners’ wishes are clear, so that WVLT can honor them as we become stewards of the property. Properties we decide to purchase require funding sources, may involve government regulation, and sometimes need months of negotiation around boundaries, minerals, and family interests. Title exams, surveys, appraisals, maps, and environmental assessments are all part of the due diligence we undertake for every project, with full review conducted by staff, our legal team, a committee of our board of directors, and ultimately by our full Board. But enough about the anatomy of a project. What really excites us is the way that WVLT can bring its land protection skills and knowledge to others, to make something positive and lasting happen. -

West Virginia Northern Flying Squirrel (WVNFS), Glauconzys Sabrinus Fuscus Five Year Status Review Appendix B — Capture Site Summaries
U.S. Fish and Wildlife Service — West Virginia Field Office West Virginia northern flying squirrel (WVNFS), Glauconzys sabrinus fuscus Five Year Status Review Appendix B — Capture Site Summaries Attached are the summaries for the 105 West Virginia northern flying squirrel (WVNFS), G.s. fuscus, capture sites l in West Virginia. Although biologists occasionally use live-trapping, nest boxes have been the primary tool for population surveys for the WVNFS. Biologists place transects of nest boxes in a survey area and check the boxes periodically for occupancy, typically twice each year, in fall and spring. Northern flying squirrels are nocturnal, leaving their nests to forage at night and returning during the day, which facilitates daytime nest box monitoring. The success of nest box monitoring relies on the squirrels occupying the boxes during the day of the survey. Menzel (2003) found that no WVNFS in her radio telemetry study used nest boxes (despite their availability) as den sites. All nests were either natural tree cavities (i.e. dens) or dreys (i.e. outside nests constructed of leaves, twigs, lichens, etc.). She also noted WVNFS used multiple den sites, switching nests on average every 3 days in summer, and utilizing up to 12 den sites per month in lesser quality habitat (Menzel 2000, Menzel et al. 2004). Further, the nest box monitoring program conducted by the DNR had a 2% average success rate of squirrel occupancy per box checked. These data confirm the difficulty of capturing squirrels via nest boxes and caution against relying on nest box survey results to determine occupied habitat, i.e., although a captured individual affirms presence, an empty nest box does not necessarily signify absence or unoccupied habitat. -

Resource Name (Heading 1)
Monongahela National Forest Forest-wide Travel Analysis Report Appendix H Monongahela National Forest Forest-Scale Roads Analysis 2003 September 2015 Appendix H 200 Sycamore Street Phone 304-636-1800 Monongahela National Forest Elkins, WV 26241 Fax 304 636 1875 Roads Analysis Report Forest Scale Roads Analysis Monongahela National Forest January 13, 2003 Version 2.0 Page 1 of 160 TABLE OF CONTENTS Page OVERVIEW OF ROADS ANALYSIS GUIDANCE…………………………………… 5 INTRODUCTION Background………………………………………………………………………. 7 Process…………………………………………………………………………… 7 Products………………………………………………………………………….. 8 This Report………………………………………………………………………. 8 STEP 1: SETTING UP THE ANALYSIS Purpose and Products……………………………………………………………. 9 Objectives of the Analysis………………………………………………………. 9 Interdisciplinary Team Members and Participants……………………………… 10 Information Needs………………………………………………………………. 10 Analysis Plan……………………………………………………………………. 10 STEP 2: DESCRIBING THE SITUATION Purpose and Products……………………………………………………………. 11 The Analysis Area………………………………………………………………. 11 Existing Road and Access System Description…………………………………. 13 Forest Plan Goal…………………………………………………………………. 17 Forest Plan Objectives…………………………………………………………… 17 Meeting Forest Plan Objectives…………………………………………………. 20 Budget……………………………………………………………………………. 22 Road Inventory…………………………………………………………………… 22 Human Population in the Analysis Area…………………………………………. 30 Road Definitions…………………………………………………………………. 45 Basic Data Needs………………………………………………………………… 50 STEP 3: IDENTIFYING ISSUES Purpose and Products……………………………………………………………. -

Georgia's Mountain Treasure Areas - 2018
Georgia's Mountain Treasure Areas - 2018 In previous Mountain Total Name Cluster Treasures Acres County Trails Hidden Creek Armuchee yes 6,429 Gordon None Johns Mountain Armuchee partially 8,451 Walker, Chattooga, Floyd Pinhoti, Keown Falls, Dry Creek Rocky Face Armuchee partially 8,624 Whitfield, Walker Pinhoti Rabun Bald Chattooga Watershed yes 17,814 Rabun Bartram, Three Forks, Pinnacle Ellicott Rock Extension Chattooga Watershed partially 3,969 Rabun None Rock Gorge Chattooga Watershed yes 3,881 Rabun Foothills Three Forks Chattooga Watershed yes 6,075 Rabun Three forks Five Falls Chattooga Watershed yes 7,488 Rabun Water Gauge, Raven Rcok Thrifts Ferry Chattooga Watershed yes 5,976 Rabun Bartram, Chattooga River Big Shoals Chattooga Watershed partially 5,635 Rabun Bartram, Willis Knob Horse Grassy Mountain Cohutta yes 9,746 Murray Windy Gap, Rocky Flats, Milma Creek Mountaintown Cohutta yes 15,604 Gimer, Fannin Benton MacKaye, Pinhoti, Bear Creek Cohutta Extensions Cohutta partially 5,239 Fannin, Murray Benton MacKaye, Pinhoti,South Fork, Horseshoe Bend Emery Creek Cohutta yes 4,277 Murray Emory Creek Buzzard Knob Northern Blue Ridge yes 9,612 Rabun, Towns None Kelly Ridge Northern Blue Ridge yes 10,408 Rabun, Towns AT Patterson Gap Northern Blue Ridge yes 5,591 Rabun None Southern Nantahala Extensions Northern Blue Ridge yes 7,200 Towns, Rabun None Andrews Cove Northern Blue Ridge partially 4,706 White, Towns AT, Andrews Cove, Rocky Mountain Brasstown Extension Northern Blue Ridge partially 5,087 Towns, Union Miller Trek High Shoals -

Ouachita Mountains Ecoregional Assessment December 2003
Ouachita Mountains Ecoregional Assessment December 2003 Ouachita Ecoregional Assessment Team Arkansas Field Office 601 North University Ave. Little Rock, AR 72205 Oklahoma Field Office 2727 East 21st Street Tulsa, OK 74114 Ouachita Mountains Ecoregional Assessment ii 12/2003 Table of Contents Ouachita Mountains Ecoregional Assessment............................................................................................................................i Table of Contents ........................................................................................................................................................................iii EXECUTIVE SUMMARY..............................................................................................................1 INTRODUCTION..........................................................................................................................3 BACKGROUND ...........................................................................................................................4 Ecoregional Boundary Delineation.............................................................................................................................................4 Geology..........................................................................................................................................................................................5 Soils................................................................................................................................................................................................6 -

Introduction to the Southern Blue Ridge Ecoregional Conservation Plan
SOUTHERN BLUE RIDGE ECOREGIONAL CONSERVATION PLAN Summary and Implementation Document March 2000 THE NATURE CONSERVANCY and the SOUTHERN APPALACHIAN FOREST COALITION Southern Blue Ridge Ecoregional Conservation Plan Summary and Implementation Document Citation: The Nature Conservancy and Southern Appalachian Forest Coalition. 2000. Southern Blue Ridge Ecoregional Conservation Plan: Summary and Implementation Document. The Nature Conservancy: Durham, North Carolina. This document was produced in partnership by the following three conservation organizations: The Nature Conservancy is a nonprofit conservation organization with the mission to preserve plants, animals and natural communities that represent the diversity of life on Earth by protecting the lands and waters they need to survive. The Southern Appalachian Forest Coalition is a nonprofit organization that works to preserve, protect, and pass on the irreplaceable heritage of the region’s National Forests and mountain landscapes. The Association for Biodiversity Information is an organization dedicated to providing information for protecting the diversity of life on Earth. ABI is an independent nonprofit organization created in collaboration with the Network of Natural Heritage Programs and Conservation Data Centers and The Nature Conservancy, and is a leading source of reliable information on species and ecosystems for use in conservation and land use planning. Photocredits: Robert D. Sutter, The Nature Conservancy EXECUTIVE SUMMARY This first iteration of an ecoregional plan for the Southern Blue Ridge is a compendium of hypotheses on how to conserve species nearest extinction, rare and common natural communities and the rich and diverse biodiversity in the ecoregion. The plan identifies a portfolio of sites that is a vision for conservation action, enabling practitioners to set priorities among sites and develop site-specific and multi-site conservation strategies.