Retinal Findings on OCT in a COVID-19 Patient Cohort
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hospital Authority Annual Plan 2005/06 I
HOSPITAL AUTHORITY ANNUAL PLAN 2005/06 Table of Contents EXECUTIVE SUMMARY 1 PLANNING BACKGROUND 1. Introduction 10 2. Review of Progress 13 3. Planning Environment 14 4. Budget Allocation 17 MAJOR DIRECTIONS AND PROGRAMME INITIATIVES FOR 2005/06 HA ANNUAL PLAN 5. Major Directions for HA Annual Plan 2005/06 20 6. Improving Population Health 22 7. Enhancing Organisational Performance 25 8. Enhancing Healthcare System Sustainability 30 9. Improving Service Quality and Clinical Governance 34 10. Building Human Resources Capability 40 CLUSTER PLANS 11. Hong Kong East Cluster 45 12. Hong Kong West Cluster 50 13. Kowloon East Cluster 54 14. Kowloon Central Cluster 58 15. Kowloon West Cluster 62 16. New Territories East Cluster 66 17. New Territories West Cluster 70 Hospital Authority Annual Plan 2005/06 i Table of Contents APPENDICES Appendix 1: List of Public Hospitals and Institutions 74 Appendix 2: List of Ambulatory Care Facilities 75 Appendix 3: Background Information on Hospital Authority 79 Appendix 4: Statistics of the Controlling Officer’s Report 81 ii Hospital Authority Annual Plan 2005/06 Executive Summary OVERVIEW 1. The Hospital Authority (HA) is responsible for delivering a comprehensive range of hospital, outpatient and community-based services through its network of healthcare facilities. As part of its commitment to enhance accountability and transparency to the community, it has been publishing its Annual Plan since 1992/93, which provides a structured mechanism for the organisation to turn its corporate vision and directions into strategies, goals and operational targets. 2. There are a number of major changes in the external and internal environment of HA that shape the major directions adopted and presented in this Annual Plan for 2005/06: (a) Key people changes after the SARS epidemic could have important bearings on the healthcare environment as well as the work of HA. -

Report of the Steering Committee on Review of Hospital Authority
Report of the Steering Committee on Review of Hospital Authority July 2015 CONTENTS Glossary .................................................................................................................. iii Executive Summary ................................................................................................ v Chapter 1 Introduction ...................................................................................... 1 Chapter 2 Work of the Steering Committee ...................................................... 6 Chapter 3 Major Challenges Facing the Hospital Authority ............................ 9 Chapter 4 Management and Organisation Structure ....................................... 13 Chapter 5 Resource Management ................................................................... 26 Chapter 6 Staff Management .......................................................................... 42 Chapter 7 Cost Effectiveness and Service Management ................................ 59 Chapter 8 Overall Management and Control .................................................. 87 Chapter 9 Conclusion ...................................................................................... 96 Annex 1 Membership of the Steering Committee on Review of Hospital Authority ....................................................................................... 102 Annex 2 Report of the Public Engagement Programme ............................. 103 Annex 3 Clustering of Hospitals and Institutions ...................................... -

Paper on Provision of Obstetric Services in the Tseung
立法會 Legislative Council LC Paper No. CB(2)486/12-13(05) Ref : CB2/PL/HS Panel on Health Services Background brief prepared by the Legislative Council Secretariat for the meeting on 21 January 2013 Provision of obstetric services in the Tseung Kwan O Hospital Purpose This paper summarizes the concerns of the members of the Panel on Health Services ("the Panel") on the provision of obstetric services in the Tseung Kwan O Hospital ("TKOH"). Background 2. The Kowloon East Cluster serves the population of Kwun Tong, Tseung Kwan O ("TKO") and Sai Kung districts which have an estimated population of 980 000. There are three hospitals in the cluster, namely, United Christian Hospital ("UCH"), TKOH and Haven of Hope Hospital. With the rapid increase of population in TKO and Sai Kung, the existing facilities in TKOH are inadequate in terms of space and capacity to meet the future service requirements and service demand in the Kowloon East Cluster. In July 2008, the Finance Committee approved a sum of $1,945 million in money-of-the-day prices for a major renovation and expansion project at TKOH. The TKOH expansion project includes, among others, the establishment of obstetric wards, Neonatal Intensive Care Unit ("NICU") as well as Special Care Baby Unit to provide the necessary facilities for the development of obstetric and neonatal services in TKOH. The whole project is targeted for completion by late 2013-2014. - 2 - 3. HA has also planned to commission the obstetric and NICU services in TKOH in phases, from enhancement of antenatal and postnatal services, delivery of low risk pregnancies to the provision of a full scale service. -

A General Brief About the Hospital Authority
Mission Statement 4. In keeping with its role, the Mission of the Hospital Authority is: · to meet the different needs of patients for public hospital services, and to improve the hospital environment for the benefit of patients; · to serve the public with care, dedication and efficiency, and to encourage community participation in the system, resulting in better care and more direct accountability to the public; · to provide rewarding, fair and challenging employment to all its staff, in an environment conducive to attracting, motivating and retaining well-qualified staff; · to advise the Government of the needs of the community for public hospital services and of the resources required to meet these needs, in order to provide adequate, efficient, effective and value for money public hospital services of the highest standards recognised internationally within the resources obtainable; and · to collaborate with other agencies and bodies in the healthcare and related fields both locally and overseas to provide the greatest benefit to the local community. Corporate Vision and Strategies 5. To realise its mission, the Hospital Authority has developed the following Corporate Vision: “The Hospital Authority will collaborate with other healthcare providers and carers in the community to create a seamless healthcare environment which will maximise healthcare benefits and meet community expectations.” 6. The Authority achieves this corporate vision by formulating a set of strategic directions every year through an extensive annual planning process, taking into account the funding position, societal expectations, Government’s healthcare policy, and the challenges in the internal and external environment. The 2 corporate vision and mission are turned into operational targets to meet the community needs for healthcare services. -
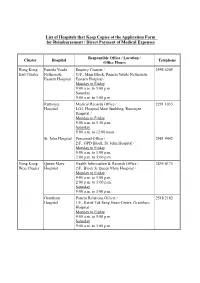
List of Hospitals That Keep Copies of the Application Form for Reimbursement / Direct Payment of Medical Expenses
List of Hospitals that Keep Copies of the Application Form for Reimbursement / Direct Payment of Medical Expenses Responsible Office / Location / Cluster Hospital Telephone Office Hours Hong Kong Pamela Youde Enquiry Counter / 2595 6205 East Cluster Nethersole G/F., Main Block, Pamela Youde Nethersole Eastern Hospital Eastern Hospital / Monday to Friday 9:00 a.m. to 5:00 p.m. Saturday 9:00 a.m. to 1:00 p.m. Ruttonjee Medical Records Office / 2291 1035 Hospital LG1, Hospital Main Building, Ruttonjee Hospital / Monday to Friday 9:00 a.m. to 5:30 p.m. Saturday 9:00 a.m. to 12:00 noon St. John Hospital Personnel Office / 2981 9442 2/F., OPD Block, St. John Hospital / Monday to Friday 9:00 a.m. to 1:00 p.m. 2:00 p.m. to 5:00 p.m. Hong Kong Queen Mary Health Information & Records Office / 2855 4175 West Cluster Hospital 2/F., Block S, Queen Mary Hospital / Monday to Friday 9:00 a.m. to 1:00 p.m. 2:00 p.m. to 5:00 p.m. Saturday 9:00 a.m. to 1:00 p.m. Grantham Patient Relations Officer / 2518 2182 Hospital 1/F., Kwok Tak Seng Heart Centre, Grantham Hospital / Monday to Friday 9:00 a.m. to 5:00 p.m. Saturday 9:00 a.m. to 1:00 p.m. - 2 - Responsible Office / Location / Cluster Hospital Telephone Office Hours Kowloon Kwong Wah Medical Report Office / 3517 5216 West Cluster Hospital 1/F., Central Stack, Kwong Wah Hospital / Monday to Friday 9:00 a.m. -

Team Leader Kowloon East Cluster United Christian Hospital
九龍東智 Smart 創新團隊 九龍東醫院聯網 Innovative KEC Smart Hospital Team Kowloon East Cluster Team Leader United Christian Hospital Deputy Hospital Chief Executive / Consultant Dr Tse Man-li (Hong Kong Poison Information Centre) Tseung Kwan O Hospital Consultant Dr Cheng Hung-kai (Anaesthesia & Operating Theatre Services) Kowloon East Cluster / United Christian Hospital Cluster General Manager / General Manager Mr Terence Cham (Administrative Services) Kowloon East Cluster Senior Human Resources Manager Associate Consultant Mr Richard To (Human Resources) Dr Wong Tseng-kwong Senior Finance Manager (Family Medicine & Primary Health Care) Mr Kwan Chi-wang (Finance) Clinical Stream Coordinator (Allied Health) / Hospital Manager TKOH Department Manager (Physiotherapy) Mr Gary Lam Mr Tony Au (Procurement & Materials Management) Clinical Stream Coordinator / Department Manager Ms Kathy Mak (Pharmacy) United Christian Hospital Deputy Chief of Service, Consultant Associate Consultant Dr Timothy Hon Yu-wai Dr Candice Liu (Ophthalmology) (Radiology & Organ Imaging) Dr Cheng James Wesley Ching-hei Consultant (Paediatric & Adolescent Medicine) Dr David Luk Dr Lam Chun-leung (Psychiatry) (Paediatrics & Adolescent Medicine) Resident Specialist Dr Anthony Njo Kui-hung Dr Lau Chi-kin (Anaesthesiology and Pain Medicine) (Orthopaedics & Traumatology) Resident Advanced Practice Nurse Dr Shasha Liu (Ophthalmology) Ms Chan Po-chun (Pain Medicine) Senior Medical Technologist Mr Kwok Wing-hang Mr Paul Huang (Pathology) (Orthopaedic & Traumatology) Pharmacist Ms Tong Suet-yan -

List of Medical Social Services Units Under Social Welfare Department
List of Medical Social Services Units Under Social Welfare Department Hong Kong Name of Hospital/Clinic Tel. No. Email Address 1. Queen Mary Hospital 2255 3762 [email protected] 2255 3764 2. Wong Chuk Hang Hospital 2873 7201 [email protected] 3. Pamela Youde Nethersole Eastern 2595 6262 [email protected] Hospital 4. Pamela Youde Nethersole Eastern 2595 6773 [email protected] Hospital (Psychiatric Department) Kowloon Name of Hospital/Clinic Tel. No. Email Address 5. Tseung Kwan O Hospital 2208 0335 [email protected] 2208 0327 6. United Christian Hospital 3949 5178 [email protected] (Psychiatry) 7. Queen Elizabeth Hospital 3506 7021 [email protected] 3506 7027 3506 5499 3506 4021 8. Hong Kong Eye Hospital 2762 3069 [email protected] 9. Kowloon Hospital Rehabilitation 3129 7857 [email protected] Building 10. Kowloon Hospital 3129 6193 [email protected] 11. Kowloon Hospital 2768 8534 [email protected] (Psychiatric Department) 1 The New Territories Name of Hospital/Clinic Tel. No. Email Address 12. Prince of Wales Hospital 3505 2400 [email protected] 13. Shatin Hospital 3919 7521 [email protected] 14. Tai Po Hospital 2607 6304 [email protected] Sub-office Tai Po Hospital (Child and Adolescent 2689 2486 [email protected] Mental Health Centre) 15. North District Hospital 2683 7750 [email protected] 16. Tin Shui Wai Hospital 3513 5391 [email protected] 17. Castle Peak Hospital 2456 7401 [email protected] 18. Siu Lam Hospital 2456 7186 [email protected] 19. -

Hospital Authority's Planned Projects for 2021-2022
LC Paper No. CB(4)503/20-21(02) Head 708 Subhead 8083MM One-Off Grant to the Hospital Authority for Minor Works Projects 2021-22 Planned Projects Prepared by the Hospital Authority February 2021 Head 708 : Subhead 8083MM One-off Grant to the Hospital Authority for Minor Works Projects for the 2019-20 Financial Year Part A - Previously approved items and other items to commence in 2020-21 with expected expenditure in 2020-21 and/or 2021-22 Actual Approved Cumulative Revised Estimated cash flow in subsequent years Expenditure Estimate Priority / Project Expenditure Estimate Project Title (1.4.2020 to 2021-22 Post Item No. Estimate to 31.3.2020 2020-21 31.10.2020) 2022-23 2023-24 2024-25 2024-25 ($'000) (I) Previously approved items (up to 31.10.2020) with expected expenditure in 2020-21 and/or 2021-22 EMR15-604 Modernisation of lifts in Day Treatment Block and Special Block in Prince of Wales Hospital 16,794 16,540 254 254 - - - - - EMR16-104 Replacement of the local central control and monitoring system for Wong Chuk Hang Hospital 1,280 1,150 39 101 - 30 - - - EMR16-401 Replacement of fire alarm and detection system at Hospital Main Block in Tseung Kwan O 6,500 6,500 (1,371) (1,371) - - - - - Hospital EMR16-504 Replacement of 1 no. main switch board for Block A in Yan Chai Hospital 2,345 2,202 142 142 - - - - - EMR16-505 Replacement of building management system at Multi Services Complex in Yan Chai Hospital 3,500 3,148 55 55 297 - - - - EMR16-506 Replacement of the air handling unit for Department of Central Supporting Services at 1/F, 502 526 (24) (24) - - - - - Block B in Yan Chai Hospital EMR17-102 Replacement of emergency generators for Hospital Block at St. -

Day Surgery in Hong Kong
The Journal of One Day Surgery | 97 Featured Day Surgery Units: Day Surgery in Hong Kong JOE KM FAN, WAI LUN LAW & WAI KEI YUEN Keywords: Day surgery facilities; International practice Background Concerning surgical training in Hong Kong, there is no designated module for day surgery. The application of day Medical infrastructure in Hong Kong is mainly surgery requires careful selection of cases and special care government funded, although charity groups subsidise by specialists. In addition, the physical setup and scale of some hospitals in addition to central funding from the day surgery facilities varies greatly among the different Government. The whole territory, which has a population hospital clusters (Figures 1 & 2): some are well equipped of eight million, is served by seven hospital clusters. with operating theatres and nurse specialist for Despite the fact that some hospitals have started a scheme perioperative care; whereas some only comprise a small of “Self-Financed Items” in which patients are required to side-ward with a few beds with nurses being deployed from pay the cost of expensive drugs, such as new other surgical wards during normal working hours. chemotherapeutic agents and special instruments for some operative procedures, an average patient pays as little as US$12 per day for in-hospital charge, which includes the room as well as all investigations and treatments during the hospital stay. The remaining cost is fully covered by the Hospital Authority of the Hong Kong Special Administrative Region (HKSAR), China. Under these circumstances, there is no apparent motivation for patients to be treated as day cases, as the cost for finding a care-giver or for travelling may be much more than staying as an inpatient for several more days. -

1 Hospitals in Hong Kong and Macau
1 Hospitals in Hong Kong and Macau ^Pre-admission approval for guarantee payment is applicable *Designated psychiatric hospital #Designated Hospital in Macau Hospital Name Address Government Hospital Pamela Youde Nethersole Eastern Hospital* 3 Lok Man Road, Chai Wan, HK Ruttonjee Hospital 266 Queen's Road East, Wan Chai, HK St. John Hospital Cheung Chau Hospital Road, Tung Wan, Cheung Chau Tang Shiu Kin Hospital 282 Queen's Road East, Wan Chai, HK Tung Wah Eastern Hospital 19 Eastern Hospital Road, Causeway Bay, HK Wong Chuk Hang Hospital 2 Wong Chuk Hang Path, Wong Chuk Hang, HK Grantham Hospital 125 Wong Chuk Hang Road, Aberdeen, HK Queen Mary Hospital* 102 Pokfulam Road, HK The Duchess of Kent Children's Hospital at Sandy Bay 12 Sandy Bay Road, Pokfulam, HK Tsan Yuk Hospital 30 Hospital Road, Sai Ying Pun, HK Tung Wah Group of Hospitals Fung Yiu King Hospital 9 Sandy Bay Road, Pokfulam, HK Tung Wah Hospital 12 Po Yan Street, Sheung Wan, HK Hong Kong Buddhist Hospital 10 Heng Lam Street, Lok Fu, KLN Hong Kong Eye Hospital 147K Argyle Street, KLN Kowloon Hospital* 147A Argyle Street, KLN Queen Elizabeth Hospital 30 Gascoigne Road, KLN Haven of Hope Hospital 8 Haven of Hope Road, Tseung Kwan O, KLN Tseung Kwan O Hospital No. 2 Po Ning Lane, Hang Hau, Tseung Kwan O, NT United Christian Hospital* 130 Hip Wo Street, Kwun Tong, KLN Caritas Medical Centre 111 Wing Hong Street, Sham Shui Po, KLN Kwai Chung Hospital* 3-15 Kwai Chung Hospital Road, NT Kwong Wah Hospital 25 Waterloo Road, KLN North Lantau Hospital 8 Chung Yan Road, Tung Chung, -

(16 January 2018) List of Hospitals/Clinics Under the Hospital Authority
Social Welfare Department List of Medical Social Services Units (16 January 2018) List of Hospitals/Clinics under the Hospital Authority Hong Kong Name of Hospital/Clinic Address Tel. No. Fax. No. Opening Hours 1. Queen Mary Hospital J122, 1/F, Block J, 2255 3762 2872 8565 Mon – Fri: 8:45 am – 5:15 pm Queen Mary Hospital, 2255 3764 Lunch break: 1:00 pm to 2:00 pm Pokfulam Road, Hong Kong Sat: 9:00 am – 12:00 noon 2. Wong Chuk Hang Hospital G/F, Wong Chuk Hang Hospital, 2873 7201 2554 7318 Mon – Fri: 8:45 am – 5:15 pm 2 Wong Chuk Hang Path, Lunch break: 12:30 pm to 1:30 pm Wong Chuk Hang, Hong Kong Sat: 9:00 am – 12:00 noon 3. Western Psychiatric Centre G/F, South Wing, David Trench 2517 8141 2559 9464 Mon – Fri: 8:30 am – 6:00 pm Rehabilitation Centre, Lunch break: 1:00 pm to 2:00 pm 1F High Street, Hong Kong 4. Pamela Youde Nethersole Eastern Room 081, 1/F, Main Block, Pamela 2595 6262 2558 6023 Mon – Fri:8:45 am – 5:15 pm Hospital Youde Nethersole Eastern Hospital, Lunch break: 1:00 pm to 2:00 pm 3 Lok Man Road, Chai Wan, Sat: 9:00 am – 1:00 pm Hong Kong 5. Pamela Youde Nethersole Eastern 7/F, East Block, Pamela Youde 2595 6773 2557 4231 Mon – Fri: 8:45 am – 5:15 pm Hospital (Psychiatric Department) Nethersole Eastern Hospital, Lunch break: 1:00 pm to 2:00 pm 3 Lok Man Road, Sat: 9:00 am – 1:00 pm Chai Wan, Hong Kong Kowloon Name of Hospital/Clinic Address Tel. -

Hospital Authority Convention 2021 Keynote Address by Dr Tony Ko Pat-Sing, Chief Executive of Hospital Authority 3 May 2021
Hospital Authority Convention 2021 Keynote Address by Dr Tony Ko Pat-sing, Chief Executive of Hospital Authority 3 May 2021 Embracing the Past, Reaching Out for a Sustainable Future Chief Executive (The Honourable Mrs Carrie Lam Cheng Yuet-ngor, GBM, GBS, Chief Executive of HKSARG), Professor Chan (Professor Sophia Chan Siu-chee, Secretary for Food and Health), Mr Fan (Mr Henry Fan Hung-ling, HA Chairman), distinguished guests, colleagues, ladies and gentlemen – good morning. 1. It is my greatest pleasure to welcome you all to the 2021 Hospital Authority (HA) Convention. 2. Last year, we reluctantly cancelled the HA Convention in the wake of the COVID-19 pandemic. This year, thanks to technology advancement and our dedicated support team, we are proud to launch this annual flagship event using a hybrid mode. Despite the limitations on cross-border travel, we are glad to have more than 50 eminent local and non-local speakers, joining us either in person or via virtual platform. I would also like to thank our Mainland and Macau counterparts who are joining us online today. My heartfelt gratitude in particular goes to Minister Ma of the National Health Commission for his thoughtful arrangement in delivering his congratulatory message to us via video. 3. This is the first HA Convention that I join after assuming the role of HA Chief Executive in 2019. I am excited to see that the Convention continues to 1 provide an excellent opportunity for colleagues and leading healthcare experts to share on a diverse array of knowledge and new ideas. 4. This year, HA is celebrating its 30th anniversary.