The Efficacy of Propylene Oxide, Ethyl Formate, and Ethanedinitrile
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bromomethane CAS #: 74-83-9 Revised By: RRD Toxicology Unit Revision Date: August 14, 2015
CHEMICAL UPDATE WORKSHEET Chemical Name: Bromomethane CAS #: 74-83-9 Revised By: RRD Toxicology Unit Revision Date: August 14, 2015 (A) Chemical-Physical Properties Part 201 Value Updated Value Reference Source Comments Molecular Weight (g/mol) 94.94 94.94 EPI EXP Physical State at ambient temp Liquid Gas MDEQ Melting Point (˚C) 179 -93.70 EPI EXP Boiling Point (˚C) 3.5 3.50 EPI EXP Solubility (ug/L) 1.45E+7 1.52E+07 EPI EXP Vapor Pressure (mmHg at 25˚C) 1672 1.62E+03 EPI EXP HLC (atm-m³/mol at 25˚C) 1.42E-2 7.34E-03 EPI EXP Log Kow (log P; octanol-water) 1.18 1.19 EPI EXP Koc (organic carbon; L/Kg) 14.5 13.22 EPI EST Ionizing Koc (L/kg) NR NA NA Diffusivity in Air (Di; cm2/s) 0.08 1.00E-01 W9 EST Diffusivity in Water (Dw; cm2/s) 8.0E-6 1.3468E-05 W9 EST CHEMICAL UPDATE WORKSHEET Bromomethane (74-83-9) Part 201 Value Updated Value Reference Source Comments Soil Water Partition Coefficient NR NR NA NA (Kd; inorganics) Flash Point (˚C) NA 194 PC EXP Lower Explosivity Level (LEL; 0.1 0.1 CRC EXP unit less) Critical Temperature (K) 467.00 EPA2004 EXP Enthalpy of Vaporization 5.71E+03 EPA2004 EXP (cal/mol) Density (g/mL, g/cm3) 1.6755 CRC EXP EMSOFT Flux Residential 2 m 2.69E-05 2.80E-05 EMSOFT EST (mg/day/cm2) EMSOFT Flux Residential 5 m 6.53E-05 6.86E-05 EMSOFT EST (mg/day/cm2) EMSOFT Flux Nonresidential 2 m 3.83E-05 4.47E-05 EMSOFT EST (mg/day/cm2) EMSOFT Flux Nonresidential 5 m 9.24E-05 1.09E-04 EMSOFT EST (mg/day/cm2) 2 CHEMICAL UPDATE WORKSHEET Bromomethane (74-83-9) (B) Toxicity Values/Benchmarks Source/Reference/ Comments/Notes Part 201 Value Updated Value Date /Issues Reference Dose 1.4E-3 2.0E-2 OPP, 2013 (RfD) (mg/kg/day) Rat subchronic Tier 1 Source: Complete gavage study EPA-OPP: (Danse et al., Basis: OPP is the more current than IRIS, PPRTV and ATSDR. -

SAFETY DATA SHEET Bromomethane (R40 B1) SECTION 1
SAFETY DATA SHEET Bromomethane (R40 B1) Issue Date: 16.01.2013 Version: 1.0 SDS No.: 000010021848 Last revised date: 02.02.2017 1/17 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier Product name: Bromomethane (R40 B1) Additional identification Chemical name: Bromomethane Chemical formula: CH3Br INDEX No. 602-002-00-2 CAS-No. 74-83-9 EC No. 200-813-2 REACH Registration No. Not available. 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses: Industrial and professional. Perform risk assessment prior to use. Using gas alone or in mixtures for the calibration of analysis equipment. Using gas as feedstock in chemical processes. Formulation of mixtures with gas in pressure receptacles. Uses advised against Consumer use. 1.3 Details of the supplier of the safety data sheet Supplier Linde Gas GmbH Telephone: +43 50 4273 Carl-von-Linde-Platz 1 A-4651 Stadl-Paura E-mail: [email protected] 1.4 Emergency telephone number: Emergency number Linde: + 43 50 4273 (during business hours), Poisoning Information Center: +43 1 406 43 43 SDS_AT - 000010021848 SAFETY DATA SHEET Bromomethane (R40 B1) Issue Date: 16.01.2013 Version: 1.0 SDS No.: 000010021848 Last revised date: 02.02.2017 2/17 SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Directive 67/548/EEC or 1999/45/EC as amended. T; R23/25 Xi; R36/37/38 Xn; R48/20 Muta. 3; R68 N; R50 N; R59 The full text for all R-phrases is displayed in section 16. -

RR Program's RCL Spreadsheet Update
RR Program’s RCL Spreadsheet Update March 2017 RR Program RCL Spreadsheet Update DNR-RR-052e The Wisconsin DNR Remediation and Redevelopment Program (RR) has updated the numerical soil standards in the August 2015 DNR-RR- 052b RR spreadsheet of residual contaminant levels (RCLs). The RCLs were determined using the U.S. EPA RSL web- calculator by accepting EPA exposure defaults, with the exception of using Chicago, IL, for the climatic zone. This documentThe U.S. provides EPA updateda summary its Regionalof changes Screening to the direct-contact Level (RSL) RCLs website (DC-RCLs) in June that2015. are To now reflect in the that March 2017 spreadsheet.update, the The Wisconsin last page ofDNR this updated document the has numerical the EPA exposuresoil standards, parameter or residual values usedcontaminant in the RCL levels calculations. (RCLs), in the Remediation and Redevelopment program’s spreadsheet of RCLs. This document The providesU.S. EPA a RSL summary web-calculator of the updates has been incorporated recently updated in the Julyso that 2015 the spreadsheet.most up-to-date There toxicity were values no changes for chemi - cals madewere certainlyto the groundwater used in the RCLs,RCL calculations. but there are However, many changes it is important in the industrial to note that and the non-industrial web-calculator direct is only a subpartcontact of the (DC) full RCLsEPA RSL worksheets. webpage, Tables and that 1 andthe other 2 of thissubparts document that will summarize have important the DC-RCL explanatory changes text, generic tablesfrom and the references previous have spreadsheet yet to be (Januaryupdated. -

A High Volume Sampling System for Isotope Determination of Volatile Halocarbons and Hydrocarbons
Atmos. Meas. Tech., 4, 2073–2086, 2011 www.atmos-meas-tech.net/4/2073/2011/ Atmospheric doi:10.5194/amt-4-2073-2011 Measurement © Author(s) 2011. CC Attribution 3.0 License. Techniques A high volume sampling system for isotope determination of volatile halocarbons and hydrocarbons E. Bahlmann, I. Weinberg, R. Seifert, C. Tubbesing, and W. Michaelis Institute for Biogeochemistry and Marine Chemistry, Hamburg, Germany Received: 15 March 2011 – Published in Atmos. Meas. Tech. Discuss.: 8 April 2011 Revised: 31 August 2011 – Accepted: 7 September 2011 – Published: 4 October 2011 Abstract. The isotopic composition of volatile organic com- 1 Introduction pounds (VOCs) can provide valuable information on their sources and fate not deducible from mixing ratios alone. Compound specific isotope ratio mass spectrometry In particular the reported carbon stable isotope ratios of (CSIRMS) of non methane volatile organic compounds chloromethane and bromomethane from different sources (NMVOCs) emerged as a powerful tool to distinguish dif- 13 cover a δ C-range of almost 100 ‰ making isotope ra- ferent sources and to provide information on sinks (Rudolph tios a very promising tool for studying the biogeochemistry et al., 1997; Rudolph and Czuba, 2000; Tsunogai et al., of these compounds. So far, the determination of the iso- 1999; Bill et al., 2002; Thompson et al., 2002; Goldstein and topic composition of C1 and C2 halocarbons others than Shaw, 2003 and references therein; Archbold et al., 2005; chloromethane is hampered by their low mixing ratios. Redeker -
Toxicological Profile for Bromomethane
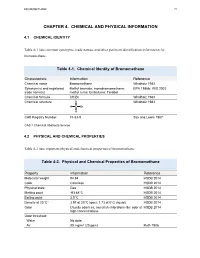
BROMOMETHANE 72 CHAPTER 4. CHEMICAL AND PHYSICAL INFORMATION 4.1 CHEMICAL IDENTITY Table 4-1 lists common synonyms, trade names, and other pertinent identification information for bromomethane. Table 4-1. Chemical Identity of Bromomethane Characteristic Information Reference Chemical name Bromomethane Windholz 1983 Synonym(s) and registered Methyl bromide; monobromomethane; EPA 1986b; IRIS 2002 trade name(s) methyl fume; Embafume; Terabol Chemical formula CH3Br Windholz 1983 Chemical structure H Windholz 1983 H C Br H CAS Registry Number 74-83-9 Sax and Lewis 1987 CAS = Chemical Abstracts Service 4.2 PHYSICAL AND CHEMICAL PROPERTIES Table 4-2 lists important physical and chemical properties of bromomethane. Table 4-2. Physical and Chemical Properties of Bromomethane Property Information Reference Molecular weight 94.94 HSDB 2014 Color Colorless HSDB 2014 Physical state Gas HSDB 2014 Melting point -93.68°C HSDB 2014 Boiling point 3.5°C HSDB 2014 Density at 20°Ca 3.97 at 20°C (gas); 1.73 at 0°C (liquid) HSDB 2014 Odor Usually odorless; sweetish chloroform-like odor at HSDB 2014 high concentrations Odor threshold: Water No data Air 80 mg/m3 (20 ppm) Ruth 1986 BROMOMETHANE 73 4. CHEMICAL AND PHYSICAL INFORMATION Table 4-2. Physical and Chemical Properties of Bromomethane Solubility: Water at 20°C 15.2 g/L at 25°C HSDB 2014 18.5 g/L at 20°C 13.4 g/L at 25°C Organic solvents Readily soluble in lower alcohols, ethers, esters, HSDB 2014 ketones, halogenated hydrocarbons, aromatic hydrocarbons, and carbon disulfide; freely soluble in benzene, carbon tetrachloride, and carbon disulfide; miscible in ethanol and chloroform Partition coefficients: Log Kow 1.19 HSDB 2014 Log Koc 0.95–1.3 Yates et al. -

Federal Register/Vol. 84, No. 230/Friday, November 29, 2019
Federal Register / Vol. 84, No. 230 / Friday, November 29, 2019 / Proposed Rules 65739 are operated by a government LIBRARY OF CONGRESS 49966 (Sept. 24, 2019). The Office overseeing a population below 50,000. solicited public comments on a broad Of the impacts we estimate accruing U.S. Copyright Office range of subjects concerning the to grantees or eligible entities, all are administration of the new blanket voluntary and related mostly to an 37 CFR Part 210 compulsory license for digital uses of increase in the number of applications [Docket No. 2019–5] musical works that was created by the prepared and submitted annually for MMA, including regulations regarding competitive grant competitions. Music Modernization Act Implementing notices of license, notices of nonblanket Therefore, we do not believe that the Regulations for the Blanket License for activity, usage reports and adjustments, proposed priorities would significantly Digital Uses and Mechanical Licensing information to be included in the impact small entities beyond the Collective: Extension of Comment mechanical licensing collective’s potential for increasing the likelihood of Period database, database usability, their applying for, and receiving, interoperability, and usage restrictions, competitive grants from the Department. AGENCY: U.S. Copyright Office, Library and the handling of confidential of Congress. information. Paperwork Reduction Act ACTION: Notification of inquiry; To ensure that members of the public The proposed priorities do not extension of comment period. have sufficient time to respond, and to contain any information collection ensure that the Office has the benefit of SUMMARY: The U.S. Copyright Office is requirements. a complete record, the Office is extending the deadline for the extending the deadline for the Intergovernmental Review: This submission of written reply comments program is subject to Executive Order submission of written reply comments in response to its September 24, 2019 to no later than 5:00 p.m. -

Risk-Based Closure Guide Draft
Risk-based Closure Guide July 1, 2021 Office of Land Quality Indiana Department of Environmental Management Disclaimer This Nonrule Policy Document (NPD) is being established by the Indiana Department of Environmental Management (IDEM) consistent with its authority under IC 13-14-1-11.5. It is intended solely as guidance and shall be used in conjunction with applicable rules or laws. It does not replace applicable rules or laws, and if it conflicts with these rules or laws, the rules or laws shall control. Pursuant to IC 13-14-1-11.5, this NPD will be available for public inspection for at least forty-five (45) days prior to presentation to the appropriate State Environmental Board, and may be put into effect by IDEM thirty (30) days afterward. If the NPD is presented to more than one board, it will be effective thirty (30) days after presentation to the last State Environmental Board. IDEM also will submit the NPD to the Indiana Register for publication. 2 Table of Contents 1 Introduction ..................................................................................................................................... 7 1.1 Applicability .............................................................................................................................. 8 1.2 Types of Closure ...................................................................................................................... 9 1.3 Process Overview ................................................................................................................... -

SAFETY DATA SHEET Methyl Bromide
SAFETY DATA SHEET Methyl Bromide Section 1. Identification GHS product identifier : Methyl Bromide Chemical name : Methyl Bromide Other means of : methylbromide; Methane, bromo-; Monobromomethane; halon-1001; Bromomthane; identification 1-Bromomethane; Methane monobrome; Methylium bromatum; Embafume; Mathane, bromo-; unsymmetrical dimethylhydrazine Product type : Gas. Product use : Synthetic/Analytical chemistry. Synonym : methylbromide; Methane, bromo-; Monobromomethane; halon-1001; Bromomthane; 1-Bromomethane; Methane monobrome; Methylium bromatum; Embafume; Mathane, bromo-; unsymmetrical dimethylhydrazine SDS # : 001035 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : FLAMMABLE GASES - Category 2 substance or mixture GASES UNDER PRESSURE - Compressed gas ACUTE TOXICITY (inhalation) - Category 2 SKIN IRRITATION - Category 2 EYE IRRITATION - Category 2A GERM CELL MUTAGENICITY - Category 2 SPECIFIC TARGET ORGAN TOXICITY (SINGLE EXPOSURE) (Respiratory tract irritation) - Category 3 SPECIFIC TARGET ORGAN TOXICITY (REPEATED EXPOSURE) - Category 2 AQUATIC HAZARD (ACUTE) - Category 1 AQUATIC HAZARD (LONG-TERM) - Category 1 HAZARDOUS TO THE OZONE LAYER - Category 1 GHS label elements Hazard pictograms : Signal word : Danger Hazard statements : Extremely flammable gas. May form explosive mixtures with air. Contains gas under pressure; may explode if heated. Fatal if inhaled. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. Suspected of causing genetic defects. May cause damage to organs through prolonged or repeated exposure. (central nervous system (CNS), kidneys) Very toxic to aquatic life with long lasting effects. -

12.6 Introduction to Mass Spectrometry
12_BRCLoudon_pgs4-4.qxd 11/26/08 9:01 AM Page 558 558 CHAPTER 12 • INTRODUCTION TO SPECTROSCOPY. INFRARED SPECTROSCOPY AND MASS SPECTROMETRY place to mount the sample in the infrared beam, and the optics and electronics necessary to measure the intensity of light absorbed or transmitted as a function of wavelength or wavenum- ber. Modern IR spectrometers, called Fourier-transform infrared spectrometers (FTIR spec- trometers), can provide an IR spectrum in a few seconds. (See Further Exploration 12.2.) The Further Exploration 12.2 FTIR Spectroscopy IR spectra in this text were obtained with an FTIR spectrometer. The sample containers (“sample cells”) used in IR spectroscopy must be made of an in- frared-transparent material. Because glass absorbs infrared radiation, it cannot be used. The conventional material used for sample cells is sodium chloride. The IR spectrum of an undi- luted (“neat”) liquid can be obtained by compressing the liquid between two optically pol- ished salt plates. If the sample is a solid, the finely ground solid can be dispersed (“mulled”) in a mineral oil and the dispersion compressed between salt plates. Alternatively, a solid can be co-fused (melted) with KBr, another IR-transparent material, to form a clear pellet. Simple presses are available to prepare KBr pellets. IR spectra can also be taken in solution cells, which consist of sodium chloride plates in appropriate holders equipped with syringe fittings for injecting the solution. When mineral-oil dispersions or solvents are used, we have to be aware of the regions in which the oil or the solvents themselves absorb IR radiation, because these absorptions inter- fere with those of the sample. -

Health Risks by Bromomethane and Other Toxic Gases in Import Cargo Ship Containers
Internat. Marit. Health, 2006, 57, 1 - 4 HEALTH RISKS BY BROMOMETHANE AND OTHER TOXIC GASES IN IMPORT CARGO SHIP CONTAINERS XAVER BAUR 1, FANG YU 1, BERND POSCHADEL 1, WIM VELDMAN 2, TOSCA KNOL-DE VOS 3 Abbreviations used: CA California GC Gas chromatography ILO International Labour Organization IMO International Maritime Organization ISPM International standard of phytosanitary measures MAC Maximum workplace concentration MS Mass spectrometry SIFT Selected ion flow tube TDS Thermal desorption system TEU Twenty foot equivalent unit 1 Institute of Occupational Medicine, Hamburg Port Health Center, University Medical Center Hamburg-Eppendorf, Germany 2 Inspectorate of the Ministry of Housing, Spatial Planing and the Environment Rotterdam, The Netherlands 3 Rijksinstitut voor Volksgezondheit en Milieu, Bilthoven, The Netherlands Address for correspondence: Xaver Baur, MD Ordinariat und Zentralinstitut für Arbeitsmedizin, Universität Hamburg Seewartenstrasse 10 D-20459 Hamburg Tel.: +49 40 428 894 500 FAX: +49 40 428 894 514 Email: [email protected] 46 TWA Time weighted average VOC Volatile organic components WHO World Health Organization ZfA Zentralinstitut fuer Arbeitsmedizin ABSTRACT Containers are increasingly used for the worldwide transport of all kinds of goods. Consistent with national and international regulations on pest controls, a growing proportion of these containers undergoes fumigation. Frequently, the prescribed labelling is missing. According to literature, this situation may lead to accidents and represents a significant health risk to dock workers, inspectors and custom workers. Furthermore, warehouse workers and even consumers may come in contact with these toxic fumigants. Presented measurement data underline this health risks due to bromomethane but also due to other fumigants and, surprisingly, due to further noxious gases. -

Methyl Bromide Last Revised — 09/26/1988
Integrated Risk Information System (IRIS) U.S. Environmental Protection Agency Chemical Assessment Summary National Center for Environmental Assessment Bromomethane; CASRN 74-83-9 Human health assessment information on a chemical substance is included in the IRIS database only after a comprehensive review of toxicity data, as outlined in the IRIS assessment development process. Sections I (Health Hazard Assessments for Noncarcinogenic Effects) and II (Carcinogenicity Assessment for Lifetime Exposure) present the conclusions that were reached during the assessment development process. Supporting information and explanations of the methods used to derive the values given in IRIS are provided in the guidance documents located on the IRIS website. STATUS OF DATA FOR Bromomethane File First On-Line 01/31/1987 Category (section) Assessment Available? Last Revised Oral RfD (I.A.) yes 09/26/1988 Inhalation RfC (I.B.) yes 04/01/1992 Carcinogenicity Assessment (II.) yes 06/01/1989 I. Chronic Health Hazard Assessments for Noncarcinogenic Effects I.A. Reference Dose for Chronic Oral Exposure (RfD) Substance Name — Bromomethane CASRN — 74-83-9 Primary Synonym — Methyl bromide Last Revised — 09/26/1988 The oral Reference Dose (RfD) is based on the assumption that thresholds exist for certain toxic effects such as cellular necrosis. It is expressed in units of mg/kg-day. In general, the RfD is an estimate (with uncertainty spanning perhaps an order of magnitude) of a daily exposure to the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious effects during a lifetime. Please refer to the Background Document for an 1 Integrated Risk Information System (IRIS) U.S. -

OLCT200 PID Application Note
OLCT200 PID Application note PID Response Factor PID Response Photoionization Detectors (PIDs) respond to a broad variety of organic gases and vapors, and some inorganic compounds. In order to get a response, the photon energy of the lamp must be greater than the ionization energy of the compound. PIDs are available with 10.6 eV lamps. This Technical Article lists the response factors for over 800 compounds with these lamps. The factors presented are inverse to the response, i.e., the higher the number the lower the response. In the table ‘ZR’ means there is no response, NA that the value has not been measured (Not Available), and NV means that the compound is not volatile enough to measure accurately even if its ionization energy is low enough. Isobutylene as Reference Gas It is always most accurate to calibrate a PID directly with the compound to be measured. However, standard gas cylinders of many chemicals are not readily available, and in such cases isobutylene is the standard reference gas used to calibrate. The response of these chemicals differs to isobutylene by the factors listed in the table below. This table allows measurement of any of the compounds in it using only isobutylene for calibration. To obtain the true concentration of the target chemical, multiply the apparent reading by the response factor listed in the table: True Concentration = Apparent Response x Response Factor For example, anisole has a response factor (RF) of 0.47 with a 10.6 eV lamp. Therefore, a reading of 10 ppm using an isobutylene-calibrated unit would correspond to: True Concentration = 10 ppm x 0.47 = 4.7 ppm Most of the Response Factors are pre-programmed into a compound library and can be called up to make the PID read out in units of the compound of interest.