CHEMICAL UPDATE WORKSHEET
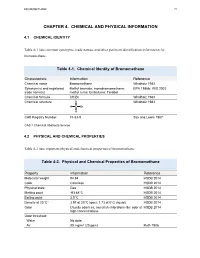
Chemical Name: CAS #:
Bromomethane 74-83-9
Revised By: Revision Date:
RRD Toxicology Unit August 14, 2015
(A) Chemical-Physical Properties
- Part 201 Value
- Updated Value
- Reference Source
- Comments
Molecular Weight (g/mol) Physical State at ambient temp Melting Point (˚C)
94.94 Liquid
179
94.94 Gas
EPI MDEQ EPI
EXP
-93.70
3.50
EXP EXP EXP EXP EXP EXP EST NA
Boiling Point (˚C)
- 3.5
- EPI
Solubility (ug/L)
1.45E+7
1672
1.42E-2
1.18
1.52E+07 1.62E+03 7.34E-03
1.19
EPI
Vapor Pressure (mmHg at 25˚C) HLC (atm-m³/mol at 25˚C) Log Kow (log P; octanol-water)
EPI EPI EPI
Koc (organic carbon; L/Kg) Ionizing Koc (L/kg)
- 14.5
- 13.22
- EPI
- NR
- NA
Diffusivity in Air (Di; cm2/s)
- 0.08
- 1.00E-01
1.3468E-05
W9 W9
EST EST
Diffusivity in Water (Dw; cm2/s)
8.0E-6
- CHEMICAL UPDATE WORKSHEET
- Bromomethane (74-83-9)
- Part 201 Value
- Updated Value
- Reference Source
- Comments
Soil Water Partition Coefficient (Kd; inorganics)
NR NA 0.1
- NR
- NA
- NA
EXP EXP EXP EXP EXP EST EST EST EST
Flash Point (˚C)
- 194
- PC
Lower Explosivity Level (LEL; unit less) Critical Temperature (K)
- 0.1
- CRC
467.00
5.71E+03
1.6755
2.80E-05 6.86E-05 4.47E-05 1.09E-04
EPA2004 EPA2004
CRC
Enthalpy of Vaporization (cal/mol) Density (g/mL, g/cm3)
EMSOFT Flux Residential 2 m (mg/day/cm2)
2.69E-05 6.53E-05 3.83E-05 9.24E-05
EMSOFT EMSOFT EMSOFT EMSOFT
EMSOFT Flux Residential 5 m (mg/day/cm2) EMSOFT Flux Nonresidential 2 m (mg/day/cm2) EMSOFT Flux Nonresidential 5 m (mg/day/cm2)
2
- CHEMICAL UPDATE WORKSHEET
- Bromomethane (74-83-9)
(B) Toxicity Values/Benchmarks
Source/Reference/ Comments/Notes
- Part 201 Value
- Updated Value
- Date
- /Issues
Reference Dose
- 1.4E-3
- 2.0E-2
- OPP, 2013
(RfD) (mg/kg/day)
Rat subchronic gavage study (Danse et al.,
Tier 1 Source: EPA-OPP:
Basis: OPP is the more current than IRIS, PPRTV and ATSDR.
Complete
1984); NOAEL=1.4 OPP chronic RfD = 0.022 mg/kg-day. mg/kg/day. UF=1000. Critical effect = epithelial
Critical Study: Mertens, J. (1997) A 24-Month Chronic Dietary Study of Methyl Bromide in Rats: Final Report: Lab Project Number: WIL-49014. Unpublished study prepared by WIL Research Labs., Inc. 4972p. (44462501) Method(s): In a combined chronic toxicity/carcinogenicity study (MRID hyperplasia of the 44462501), microencapsulated methyl bromide was administered to 4 groups of
- forestomach.
- male and female Crl:CD®(SD)BR rats for a period of 12 or 24 months (interim and
main study, respectively) in the diet at concentrations of 0 (diet control), 0 (placebo control), 0.5, 2.5, 50, or 250 ppm (equivalent to 0, 0.02, 0.11, 2.20 and 11.10 mg/kg/day in males and 0, 0.03, 0.15, 2.92 and 15.12 mg/kg/day in females. Groups of 50 males and 50 females were designated for the main study and were treated for up to 104 weeks. Groups of 20 males and 20 females were sacrificed at 52 weeks in the diet control, placebo control, 50 ppm group and the 250 ppm group.
Entry date: 5/26/1988
RfD details
Critical effect: decreased body weight gain and food consumption in males
End point or Point of Departure (POD): NOAEL = 2.2 mg/kg-day (50 ppm) for
males Uncertainty Factors: UF = 100 (10 each for intraspecies variability and interspecies extrapolation) Source and date: EPA-OPP, 9/13/2013. IRIS (2015) refers to OPP for toxicity updates of methylbromide.
Tier 1 and 2 Sources: EPA:OPP: Other OPP values:
Per EPA-OPP (9/13/2013),
1) acute RfD for females ages 13-50 years = 0.014 (1.4E-2) mg/kg-day:
3
- CHEMICAL UPDATE WORKSHEET
- Bromomethane (74-83-9)
Source/Reference/ Comments/Notes
- Part 201 Value
- Updated Value
- Date
- /Issues
Critical Study (ies): Breslin, W.; Zablotny, C.; Bradley, G. (1990) Methyl Bromide Inhalation Teratology Study in New Zealand White Rabbits: Lab Project Number: K-000681-033. Unpublished study prepared by The Dow Chemical Co. 362 p. (41580401) Method(s): rabbit developmental study: pregnant New Zealand White rabbits (26 animals/dose) were exposed by whole body inhalation to 0, 20, 40 or 80 ppm MeBr vapor for 6 hr./day on Days 6-16 of gestation. Mating was conducted using artificial insemination. Critical effect: developmental toxicity – agenesis (absence) of the gall bladder in the fetus and increased incidence of fused sternebrae
End point or Point of Departure (POD): NOAEL = 40 ppm (14 mg/kg-day)
Uncertainty Factors: UF = 100 (10 each for intraspecies variability and interspecies extrapolation); FQPA SF = 1X
2) acute RfD for general population including infants and children = 0.9 mg/kg-day:
Critical Study (ies): Driscoll, C.; Hurley, J. (1993) Methyl Bromide: Single Exposure Vapor Inhalation Neurotoxicity Study in Rats: Lab Project Number: 92N1197. Unpublished study prepared by Union Carbide, 558 p. May 27, 1993. (42793601), Method(s): acute rat neurotoxicity study: CD rats (15 rats/sex/dose) were exposed by whole body inhalation to 0, 30, 100 or 350 ppm MeBr vapor for 6 hours (equivalent to males: 0, 27, 90 or 314 mg/kg/day and females: 0, 30, 101, or 354 mg/kg/day). Critical effect: decreased activity, increase in number of animals with drooping/half-closed eyelids and alertness, decreased rears, decreased motor activity, increased piloerection and decreased body temperature
End point or Point of Departure (POD): NOAEL = 100 ppm (90 mg/kg-day)
Uncertainty Factors: UF = 100 (10 each for intraspecies variability and interspecies extrapolation); FQPA SF = 1X
4
- CHEMICAL UPDATE WORKSHEET
- Bromomethane (74-83-9)
Source/Reference/ Comments/Notes
- Part 201 Value
- Updated Value
- Date
- /Issues
IRIS: Per IRIS (7/1/1991), RfD = 1.4E-3 mg/kg-day Critical Study: Danse, L.H.J.C., F.L. van Velsen and C.A. van der Heijden. 1984. Methylbromide: Carcinogenic effects in the rat forestomach. Toxicol. Appl. Pharmacol. 72: 262-271). Method(s): Wistar rats (10/sex/dose) were exposed to 0, 0.4, 2, 10, or 50 mg/kg by gavage for 5 days/week for 13 weeks Critical effect: epithelial hyperplasia in the forestomach
End point or Point of Departure (POD): NOAEL = 2.0 mg/kg; schedule-adjusted
NOAEL = 1.4 mg/kg/day Uncertainty Factors: UF = 1,000 (10 each for intraspecies variability, interspecies extrapolation and use of a subchronic study) Source and date: IRIS, Last revision date - 7/1/1991. An IRIS screening-level review in 2001 identified one or more significant new studies.
PPRTV: Per PPRTV (6/5/2007), subchronic p-RfD = 5.0E-3 mg/kg-day Critical Study: Danse, L.H.J.C., F.L. van Velsen and C.A. van der Heijden. 1984. Methylbromide: Carcinogenic effects in the rat forestomach. Toxicol. Appl. Pharmacol. 72: 262-271). Method(s): Wistar rats (10/sex/dose) were exposed to 0, 0.4, 2, 10, or 50 mg/kg by gavage for 5 days/week for 13 weeks Critical effect: forestomach hyperplasia in rats
End point or Point of Departure (POD): NOAEL = 2 mg/kg; NOAEL(ADJ) = 1.4
mg/kg-day Uncertainty Factors: UF = 300 (10 each for intraspecies variability and interspecies extrapolation, and 3 for database deficiencies)
Source and date: PPRTV, 6/5/2007
MRL: Per ATSDR (9/1992), no chronic oral MRL at this time. An oral intermediate MRL = 0.003 mg/kg-day is available: From 4/2015 MRL list. Critical Study (ies): Danse, L.H.J.C., F.L. van Velsen and C.A. van der Heijden. 1984. Methylbromide: Carcinogenic effects in the rat forestomach. Toxicol. Appl.
5
- CHEMICAL UPDATE WORKSHEET
- Bromomethane (74-83-9)
Source/Reference/ Comments/Notes
- Part 201 Value
- Updated Value
- Date
- /Issues
Pharmacol. 72: 262-271). Method(s): Bromomethane was administered to Wistar rats (10/sex/dose) by gavage 5 days/week for 13 weeks at 0, 0.4, 2, 10, or 50 mg/kg.
Critical effect: hyperplasia and ulcers End point or Point of Departure (POD): adjusted NOAEL = 0.4 mg/kg-day
Uncertainty Factors: UF = 100 (10 each for intraspecies variability and interspecies extrapolation)
Tier 3 Source:
MDEQ: Per DEQ-CCD/RRD (5/26/1988), RfD = 1.4E-3 mg/kg-day. See Part 201 RfD details.
Oral Cancer Slope Factor (CSF)
- --
- NA
- MDEQ, 2015
(mg/kg-day)-1)
Tier 1 and 2 Sources:
IRIS: Per IRIS (1989), bromomethane is classified as D (not classifiable as to human carcinogenicity) based on inadequate human and animal data: a single mortality study from which direct exposure associations could not be deduced and studies in several animal species with too few animals, too brief exposure or observation time for adequate power.
Complete
EPA-OPP: Per OPP (2013), methyl bromide is classified as “Not Likely to be
Carcinogenic to Humans" based on a weight of evidence evaluation of the toxicity database including no indications of carcinogenesis observed in the chronic rodent bioassays.
CSF details
NA
PPRTV: Per PPRTV (6/05/07), there is inadequate information to assess the carcinogenic potential of bromomethane in humans. MRL: NA; MRLs are for non-cancer effects only.
Tier 3 Source:
MDEQ: Per DEQ-CCD, no value at this time.
Reference Concentration (RfC) or Initial
- 5.0E+0
- 1.0E+1
- PPRTV, 2007
6
- CHEMICAL UPDATE WORKSHEET
- Bromomethane (74-83-9)
Source/Reference/ Comments/Notes
- Part 201 Value
- Updated Value
- Date
- /Issues
Threshold Screening Level (ITSL) (µg/m³)
Tier 2 Source: PPRTV:
Basis: PPRTV (6/5/2007) is the more current than IRIS. MDEQ applied an additional UF of 10 to account for sub chronic to chronic to derive the chronic RfC = 1.0E-2 mg/m3.
Complete
ATSDR subchronic p-RfC = 1.0E-1 mg/m3. Critical Study: NTP (National Toxicology Program). 1992. Toxicology and carcinogenesis studies of methyl bromide (CAS No. 74-83-9) in B6C3F1 mice (inhalation studies). NTP TR 385, NIH Publication No. 92-2840. Method(s): A 13-week subchronic inhalation range-finding study was conducted in F344 rats (18/sex/group) exposed to target concentrations of 0, 30, 60 or 120 ppm (0, 116, 233 or 466 mg/m3) of bromomethane 6 hours/day, 5 days/week.
Based on EPA's RfC of 5 ug/m3, from Reuzel et al 1987 & 1991 - rat Additional groups of eight rats of each sex were exposed for eurobehavioral 29 month inhalation LOAEL of 3 ppm. studies. Critical effect: degeneration of the olfactory epithelium of the nasal cavity
End point or Point of Departure (POD): NOAEL = 233 mg/m3; NOAELHEC = 4.0
mg/m3
RfC/ITSL details
Olfactory epithelium lesions Uncertainty Factors: UF = 30 (10 each for intraspecies variability and 3 for were observed. CCD/AQD date: 12/10/1991 interspecies extrapolation)
Source and date: PPRTV, 6/5/2007 Tier 1 and 2 Sources:
IRIS: IRIS (10/1/1992) RfC = 5.0E-3 mg/m3:
Critical Study(ies):
1) Reuzel, P.G.J., C.F. Kuper, H.C. Dreef-van der Meulen and V.M.H. Hollanders. 1987. Chronic (29-month) inhalation toxicity and carcinogenicity study of methyl bromide in rats. Report No. V86.469/221044. Netherlands Organization for Applied Scientific Research, Division for Nutrition and Food Research, TNO. EPA/OTS Document No. 86-8700001202. 2) Reuzel, P.G.J., H.C. Dreef-van der Meulen, V.M.H. Hollanders, C.F. Kuper, V.J.
7
- CHEMICAL UPDATE WORKSHEET
- Bromomethane (74-83-9)
Source/Reference/ Comments/Notes
- Part 201 Value
- Updated Value
- Date
- /Issues
Feron and C.A. van der Heijden. 1991. Chronic inhalation toxicity and carcinogenicity study of methyl bromide in Wistar rats. Fd. Chem. Toxic. 29(1): 31- 39. Method(s): 50 male and 60 female Wistar rats were exposed to 0, 3, 30, or 90 ppm (0, 11.7, 117, or 350 mg/cu.m, respectively) bromomethane for 6 hours/day, 5 days/week (duration- adjusted concentrations are 0, 2.08, 20.9, or 62.5 mg/m3, respectively) for up to 29 months. Three satellite groups of 10/sex/group were sacrificed at 14, 53, and 105 weeks of exposure. Critical effect: Degenerative and proliferative lesions of the olfactory epithelium of the nasal cavity
End point or Point of Departure (POD): LOAEL = 3 ppm (11.7 mg/m3); LOAELHEC
0.48 mg/m3)
=
Uncertainty Factors: UF = 100 (10 each for intraspecies variability, a factor of 3 for the use of a LOAEL for a mild effects and 3 for interspecies extrapolation) Source and date: IRIS, Last revision date - 10/1/1992. An IRIS screening-level review conducted in 2001 identified one or more significant new studies.
MRL: Per ATSDR (9/1992), chronic inhalation MRL = 0.005 ppm (2.0 E-2 mg/m3) based on neurotoxic effects. From 4/2015 MRL list. Critical Study: Anger, W.K., L. Moody, J. Burg et al. 1986. Neurobehavioral evaluation of soil and structural fumigators using methyl bromide and sulfuryl fluoride. Neurotoxicology. 7(3): 137-156. Method(s): epidemiological study of workers who had an increased prevalence of muscle ache, fatigue, and ataxia following 8-yearchronic exposure to average levels of 2.3 ppm. Critical effect: increased prevalence of muscle ache, fatigue, and ataxia
End point or Point of Departure (POD): LOAEL = 2.3 ppm
Uncertainty Factors: UF = 100 (10 each for intraspecies variability and use of a LOAEL)
Per ATSDR (9/1992) an intermediate-duration inhalation MRL = 0.05 ppm is also derived. From 4/2015 MRL list:
8
- CHEMICAL UPDATE WORKSHEET
- Bromomethane (74-83-9)
Source/Reference/ Comments/Notes
- Part 201 Value
- Updated Value
- Date
- /Issues
Critical Study: Honma T, A. Sudo, M. Miyagawa et al. 1982. Significant changes in monoamines in rat brain induced by exposure to methyl bromide. Neurobehav. Toxicol. Teratol. 4: 521-524.
Method(s): 3-week study in rats
Critical effect: decreased brain neurotransmitters
End point or Point of Departure (POD): NOAEL = 5 ppm
Uncertainty Factors: UF = 100 (10 each for intraspecies variability and use of a LOAEL)
Tier 3 Source:
MDEQ: Per DEQ-CCD/AQD (12/10/1991), ITSL = 5.0 ug/m3. See Part 201 RfC details.
Inhalation Unit Risk Factor
- --
- NA
- MDEQ, 2015
(IURF) ((µg/m3)-1)
Per IRIS (1989), bromomethane is classified as D (not classifiable as to human carcinogenicity) based on inadequate human and animal data: a single mortality study from which direct exposure associations could not be deduced and studies in several animal species with too few animals, too brief exposure or observation time for adequate power.
Complete
Per OPP (2013), methyl bromide is classified as “Not Likely to be Carcinogenic to
Humans" based on a weight of evidence evaluation of the toxicity database including no indications of carcinogenesis observed in the chronic rodent bioassays.
IURF details
NA
Tier 1 and 2 Sources: IRIS: No value available. EPA-OPP: No value available.
PPRTV: Per PPRTV (6/05/07), there is inadequate information to assess the carcinogenic potential of bromomethane in humans. MRL: NA; MRLs are for non-cancer effects only.
Tier 3 Source:
9
- CHEMICAL UPDATE WORKSHEET
- Bromomethane (74-83-9)
Source/Reference/ Comments/Notes
- Part 201 Value
- Updated Value
- Date
- /Issues
MDEQ: Per DEQ-CCD/AQD, no value at this time.
Mutagenic Mode of Action (MMOA)? (Y/N)
-- --
- NO
- USEPA, 2015
NA
MMOA Details
Not listed as a carcinogen with mutagenic MOA in the USEPA OSWER List.
No, the RfD or RfC/ITSL is not based on a reproductive-