Acceptable Ambient Level (AAL) Recommendation for Methyl Bromide North Carolina Department of Environmental Quality August 2018
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Bromomethane CAS #: 74-83-9 Revised By: RRD Toxicology Unit Revision Date: August 14, 2015
CHEMICAL UPDATE WORKSHEET Chemical Name: Bromomethane CAS #: 74-83-9 Revised By: RRD Toxicology Unit Revision Date: August 14, 2015 (A) Chemical-Physical Properties Part 201 Value Updated Value Reference Source Comments Molecular Weight (g/mol) 94.94 94.94 EPI EXP Physical State at ambient temp Liquid Gas MDEQ Melting Point (˚C) 179 -93.70 EPI EXP Boiling Point (˚C) 3.5 3.50 EPI EXP Solubility (ug/L) 1.45E+7 1.52E+07 EPI EXP Vapor Pressure (mmHg at 25˚C) 1672 1.62E+03 EPI EXP HLC (atm-m³/mol at 25˚C) 1.42E-2 7.34E-03 EPI EXP Log Kow (log P; octanol-water) 1.18 1.19 EPI EXP Koc (organic carbon; L/Kg) 14.5 13.22 EPI EST Ionizing Koc (L/kg) NR NA NA Diffusivity in Air (Di; cm2/s) 0.08 1.00E-01 W9 EST Diffusivity in Water (Dw; cm2/s) 8.0E-6 1.3468E-05 W9 EST CHEMICAL UPDATE WORKSHEET Bromomethane (74-83-9) Part 201 Value Updated Value Reference Source Comments Soil Water Partition Coefficient NR NR NA NA (Kd; inorganics) Flash Point (˚C) NA 194 PC EXP Lower Explosivity Level (LEL; 0.1 0.1 CRC EXP unit less) Critical Temperature (K) 467.00 EPA2004 EXP Enthalpy of Vaporization 5.71E+03 EPA2004 EXP (cal/mol) Density (g/mL, g/cm3) 1.6755 CRC EXP EMSOFT Flux Residential 2 m 2.69E-05 2.80E-05 EMSOFT EST (mg/day/cm2) EMSOFT Flux Residential 5 m 6.53E-05 6.86E-05 EMSOFT EST (mg/day/cm2) EMSOFT Flux Nonresidential 2 m 3.83E-05 4.47E-05 EMSOFT EST (mg/day/cm2) EMSOFT Flux Nonresidential 5 m 9.24E-05 1.09E-04 EMSOFT EST (mg/day/cm2) 2 CHEMICAL UPDATE WORKSHEET Bromomethane (74-83-9) (B) Toxicity Values/Benchmarks Source/Reference/ Comments/Notes Part 201 Value Updated Value Date /Issues Reference Dose 1.4E-3 2.0E-2 OPP, 2013 (RfD) (mg/kg/day) Rat subchronic Tier 1 Source: Complete gavage study EPA-OPP: (Danse et al., Basis: OPP is the more current than IRIS, PPRTV and ATSDR. -

SAFETY DATA SHEET Bromomethane (R40 B1) SECTION 1
SAFETY DATA SHEET Bromomethane (R40 B1) Issue Date: 16.01.2013 Version: 1.0 SDS No.: 000010021848 Last revised date: 02.02.2017 1/17 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier Product name: Bromomethane (R40 B1) Additional identification Chemical name: Bromomethane Chemical formula: CH3Br INDEX No. 602-002-00-2 CAS-No. 74-83-9 EC No. 200-813-2 REACH Registration No. Not available. 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses: Industrial and professional. Perform risk assessment prior to use. Using gas alone or in mixtures for the calibration of analysis equipment. Using gas as feedstock in chemical processes. Formulation of mixtures with gas in pressure receptacles. Uses advised against Consumer use. 1.3 Details of the supplier of the safety data sheet Supplier Linde Gas GmbH Telephone: +43 50 4273 Carl-von-Linde-Platz 1 A-4651 Stadl-Paura E-mail: [email protected] 1.4 Emergency telephone number: Emergency number Linde: + 43 50 4273 (during business hours), Poisoning Information Center: +43 1 406 43 43 SDS_AT - 000010021848 SAFETY DATA SHEET Bromomethane (R40 B1) Issue Date: 16.01.2013 Version: 1.0 SDS No.: 000010021848 Last revised date: 02.02.2017 2/17 SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Directive 67/548/EEC or 1999/45/EC as amended. T; R23/25 Xi; R36/37/38 Xn; R48/20 Muta. 3; R68 N; R50 N; R59 The full text for all R-phrases is displayed in section 16. -

Fluoride (Developmental Neurotoxicity and Endocrine Disruption)
Bill Osmunson DDS, MPH November 6, 2015 [email protected] Dr. Kristina Thayer Director, Office of Health Assessment and Translation National Toxicology Program Email: [email protected] (1) DATA ON CURRENT PRODUCTION, USE PATTERNS, AND HUMAN EXPOSURE A. The FDA approved fluoride toothpaste caution, “Do Not Swallow.” No safe dosage or amount is stated. “Do Not Swallow” is simple to understand. Use a pea or smear size and if more than used for brushing is swallowed, contact the poison control center. A quarter milligram of fluoride is in a pea size of fluoride toothpaste, the same amount found in each 11 oz glass of 0.7 ppm fluoridated public water. Fluoridated water is dispensed to everyone without regards for other fluoride exposures, individual consent, health, or sensitivity. The only safe amount of fluoride is, “Do Not Swallow.” Swallowing fluoride is not safe, however, many children swallow their toothpaste due to taste and the swallow reflex is part of the spitting action. B. Fluoridated water is the primary source of fluoride for many people. Fluoridated toothpaste is considered the second primary source and for some is more than fluoridated water. Other significant sources of fluoride include bone meal, mechanically deboned meat, grape products with cryolite, several legend drugs and sulfuryl fluoride, a post harvest fumigant. C. The FDA sent a letter to about 35 fluoride supplement manufacturers, “. there is no substantial evidence of drug effectiveness as prescribed, recommended or suggested in its labeling. .” (Drug Therapy -

Residual Fluoride in Food Fumigated with Sulfuryl Fluoride
Fluoride 2005;38(3):175–177 Editorial 175 RESIDUAL FLUORIDE IN FOOD FUMIGATED WITH SULFURYL FLUORIDE SUMMARY: New US federal allowances for inorganic fluoride residues in food fumigated with sulfuryl fluoride are excessively high and are at levels known to cause serious adverse health effects, including crippling skeletal fluorosis. Fluoride in food has long been recognized as a potential source of adverse health effects including dental fluorosis, bone and joint defects, gastrointestinal disturbances, and muscular-neurological disorders,1-2 plus even crippling skeletal fluorosis.3 In China, food contaminated by fluoride (F) from unvented coal burning used to dry and preserve grains and other crops is an important source of F intake,4 and F levels from such exposure ranging from 18 to 87 ppm in corn and 114 to 1109 ppm in chili have been reported.5 In these coal-burning F-endemic areas, even with very little F in the drinking water, the total daily F intake by affected adults has been estimated to be between 6.12 and 9.65 mg, with the major contribution coming from food.5 By contrast, in non-endemic areas, daily F intake was estimated to be only 0.8 mg/day.5 Therefore, any appreciable increase in F intake resulting from commercial food fumigation procedures can only be viewed with grave concern. In place of methyl bromide as an insecticide fumigant because of its upper atmosphere ozone depleting ability, a number of substitutes are already in use with varying results. For example, various combinations of phosphine (PH3) and carbon dioxide have been fairly successful for food as well as general fumigation, although equipment corrosion from phosphine and insect resistance to it are among its drawbacks. -

( 12 ) United States Patent
US009853326B2 (12 ) United States Patent ( 10 ) Patent No. : US 9 ,853 , 326 B2 Tokuda et al. ( 45 ) Date of Patent: Dec. 26 , 2017 ( 54 ) NONAQUEOUS ELECTROLYTE FOR (58 ) Field of Classification Search SECONDARY BATTERY AND CPC .. .. HO1M 10 /0569 ; HOTM 10 /0567 ; HOLM NONAQUEOUS - ELECTROLYTE 10 /0525 ; HOTM 4 /587 ; HOTM 4 / 134 ; SECONDARY BATTERY EMPLOYING THE HO1M 2300 /0025 ; YO2E 60 / 122 SAME See application file for complete search history . References Cited ( 71 ) Applicant: MITSUBISHI CHEMICAL (56 ) CORPORATION , Chiyoda- ku ( JP ) U . S . PATENT DOCUMENTS 5 ,580 ,684 A 12 / 1996 Yokoyama et al . (72 ) Inventors : Hiroyuki Tokuda, Ibaraki ( JP ) ; 5 , 754 , 393 A 5 / 1998 Hiratsuka et al. Minoru Kotato , Ibaraki ( JP ) ; Masahiro 6 ,001 , 325 A 12 / 1999 Salmon et al. Takehara , Ibaraki ( JP ) ; Shinichi 6 ,033 , 808 A 3 / 2000 Salmon et al. Kinoshita , Ibaraki ( JP ) 6 , 436, 582 B1 8 / 2002 Hamamoto et al. 6 , 919, 145 B1 7 / 2005 Kotato et al . 2001/ 0044051 AL 11/ 2001 Hamamoto et al. ( 73 ) Assignee : MITSUBISHI CHEMICAL 2002 / 0192564 Al 12 /2002 Ota et al. CORPORATION , Chiyoda -ku ( JP ) 2004 / 0048164 Al 3 /2004 Jung et al . 2004 / 0091786 A1 5 / 2004 Unoki et al. 2004 / 0191636 A1 9 / 2004 Kida et al . ( * ) Notice: Subject to any disclaimer , the term of this 2005 / 0008939 A1 1 / 2005 Ota et al. patent is extended or adjusted under 35 2005 /0014071 A1 1 / 2005 Noda et al. U . S . C . 154 ( b ) by 412 days. 2005 / 0084765 A1 4 / 2005 Lee et al . 2005 / 0118512 Al 6 / 2005 Onuki et al . -
RR Program's RCL Spreadsheet Update
RR Program’s RCL Spreadsheet Update March 2017 RR Program RCL Spreadsheet Update DNR-RR-052e The Wisconsin DNR Remediation and Redevelopment Program (RR) has updated the numerical soil standards in the August 2015 DNR-RR- 052b RR spreadsheet of residual contaminant levels (RCLs). The RCLs were determined using the U.S. EPA RSL web- calculator by accepting EPA exposure defaults, with the exception of using Chicago, IL, for the climatic zone. This documentThe U.S. provides EPA updateda summary its Regionalof changes Screening to the direct-contact Level (RSL) RCLs website (DC-RCLs) in June that2015. are To now reflect in the that March 2017 spreadsheet.update, the The Wisconsin last page ofDNR this updated document the has numerical the EPA exposuresoil standards, parameter or residual values usedcontaminant in the RCL levels calculations. (RCLs), in the Remediation and Redevelopment program’s spreadsheet of RCLs. This document The providesU.S. EPA a RSL summary web-calculator of the updates has been incorporated recently updated in the Julyso that 2015 the spreadsheet.most up-to-date There toxicity were values no changes for chemi - cals madewere certainlyto the groundwater used in the RCLs,RCL calculations. but there are However, many changes it is important in the industrial to note that and the non-industrial web-calculator direct is only a subpartcontact of the (DC) full RCLsEPA RSL worksheets. webpage, Tables and that 1 andthe other 2 of thissubparts document that will summarize have important the DC-RCL explanatory changes text, generic tablesfrom and the references previous have spreadsheet yet to be (Januaryupdated. -

A High Volume Sampling System for Isotope Determination of Volatile Halocarbons and Hydrocarbons
Atmos. Meas. Tech., 4, 2073–2086, 2011 www.atmos-meas-tech.net/4/2073/2011/ Atmospheric doi:10.5194/amt-4-2073-2011 Measurement © Author(s) 2011. CC Attribution 3.0 License. Techniques A high volume sampling system for isotope determination of volatile halocarbons and hydrocarbons E. Bahlmann, I. Weinberg, R. Seifert, C. Tubbesing, and W. Michaelis Institute for Biogeochemistry and Marine Chemistry, Hamburg, Germany Received: 15 March 2011 – Published in Atmos. Meas. Tech. Discuss.: 8 April 2011 Revised: 31 August 2011 – Accepted: 7 September 2011 – Published: 4 October 2011 Abstract. The isotopic composition of volatile organic com- 1 Introduction pounds (VOCs) can provide valuable information on their sources and fate not deducible from mixing ratios alone. Compound specific isotope ratio mass spectrometry In particular the reported carbon stable isotope ratios of (CSIRMS) of non methane volatile organic compounds chloromethane and bromomethane from different sources (NMVOCs) emerged as a powerful tool to distinguish dif- 13 cover a δ C-range of almost 100 ‰ making isotope ra- ferent sources and to provide information on sinks (Rudolph tios a very promising tool for studying the biogeochemistry et al., 1997; Rudolph and Czuba, 2000; Tsunogai et al., of these compounds. So far, the determination of the iso- 1999; Bill et al., 2002; Thompson et al., 2002; Goldstein and topic composition of C1 and C2 halocarbons others than Shaw, 2003 and references therein; Archbold et al., 2005; chloromethane is hampered by their low mixing ratios. Redeker -

Sound Management of Pesticides and Diagnosis and Treatment Of
* Revision of the“IPCS - Multilevel Course on the Safe Use of Pesticides and on the Diagnosis and Treatment of Presticide Poisoning, 1994” © World Health Organization 2006 All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. CONTENTS Preface Acknowledgement Part I. Overview 1. Introduction 1.1 Background 1.2 Objectives 2. Overview of the resource tool 2.1 Moduledescription 2.2 Training levels 2.3 Visual aids 2.4 Informationsources 3. Using the resource tool 3.1 Introduction 3.2 Training trainers 3.2.1 Organizational aspects 3.2.2 Coordinator’s preparation 3.2.3 Selection of participants 3.2.4 Before training trainers 3.2.5 Specimen module 3.3 Trainers 3.3.1 Trainer preparation 3.3.2 Selection of participants 3.3.3 Organizational aspects 3.3.4 Before a course 4. -

California Restricted Materials Requirements (English)
CALIFORNIA RESTRICTED MATERIALS REQUIREMENTS FEDERAL RESTRICTED USE PESTICIDES RESTRICTED USE PESTICIDE A (Included by reference as California Restricted Materials) DUE TO (reason for restricted use classification) Pesticides display the RESTRICTED USE PESTICIDE (RUP) statement on For retail sale to and use only by Certified Applicators or the pesticide container similar to the statement shown here. RUPs require an persons under their direct supervision and only for those RUP statement enclosed in a box, at the top of the front panel of the label. uses covered by the Certified Applicator's certification. Some product labels require a Certified Applicator be “physically present” at the use site. B CALIFORNIA RESTRICTED MATERIALS This section is written in a quick reference format; refer to Title 3, California Code of Regulations (3 CCR) section 6400 for complete text. Acrolein, labeled for use as an aquatic Chlorpyrifos, labeled for the Metam sodium, labeled for the Potassium n-methyldithiocarbamate herbicide production of an agricultural production of agricultural plant (metam-potassium), labeled for the Aldicarb – unregistered commodity commodities production of agricultural plant All dust (except products containing Dazomet, labeled for the production Methamidophos – unregistered commodities only exempt pesticides)** of agricultural plant commodities Methidathion Propanil (3,4-dichloropropionanilide) Aluminum phosphide Dicamba* Methomyl†† Sodium cyanide Any pesticide containing active 2,4-dichlorophenoxyacetic acid Methyl bromide Sodium -
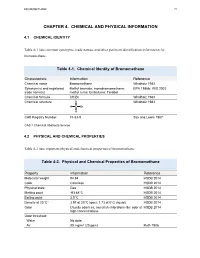
Toxicological Profile for Bromomethane
BROMOMETHANE 72 CHAPTER 4. CHEMICAL AND PHYSICAL INFORMATION 4.1 CHEMICAL IDENTITY Table 4-1 lists common synonyms, trade names, and other pertinent identification information for bromomethane. Table 4-1. Chemical Identity of Bromomethane Characteristic Information Reference Chemical name Bromomethane Windholz 1983 Synonym(s) and registered Methyl bromide; monobromomethane; EPA 1986b; IRIS 2002 trade name(s) methyl fume; Embafume; Terabol Chemical formula CH3Br Windholz 1983 Chemical structure H Windholz 1983 H C Br H CAS Registry Number 74-83-9 Sax and Lewis 1987 CAS = Chemical Abstracts Service 4.2 PHYSICAL AND CHEMICAL PROPERTIES Table 4-2 lists important physical and chemical properties of bromomethane. Table 4-2. Physical and Chemical Properties of Bromomethane Property Information Reference Molecular weight 94.94 HSDB 2014 Color Colorless HSDB 2014 Physical state Gas HSDB 2014 Melting point -93.68°C HSDB 2014 Boiling point 3.5°C HSDB 2014 Density at 20°Ca 3.97 at 20°C (gas); 1.73 at 0°C (liquid) HSDB 2014 Odor Usually odorless; sweetish chloroform-like odor at HSDB 2014 high concentrations Odor threshold: Water No data Air 80 mg/m3 (20 ppm) Ruth 1986 BROMOMETHANE 73 4. CHEMICAL AND PHYSICAL INFORMATION Table 4-2. Physical and Chemical Properties of Bromomethane Solubility: Water at 20°C 15.2 g/L at 25°C HSDB 2014 18.5 g/L at 20°C 13.4 g/L at 25°C Organic solvents Readily soluble in lower alcohols, ethers, esters, HSDB 2014 ketones, halogenated hydrocarbons, aromatic hydrocarbons, and carbon disulfide; freely soluble in benzene, carbon tetrachloride, and carbon disulfide; miscible in ethanol and chloroform Partition coefficients: Log Kow 1.19 HSDB 2014 Log Koc 0.95–1.3 Yates et al. -

Pesticides EPA-738-F-93-012 Environmental Protection and Toxic Substances September 1993 Agency (7508W) R.E.D
RED Facts Sulfuryl Fluoride September 1993 The information contained in the following document was accurate at the time of publication, but may not reflect EPA's current scientific understanding of this pesticide. For more information about this pesticide, please contact EPA's pesticide Registration Division at 703-305-5447. More current information on the regulatory status of this pesticide is available in the following documents: Sulfuryl Fluoride, Pesticide Tolerance; Federal Register; January 23, 2004 http://www.epa.gov/EPA-PEST/2004/January/Day-23/p1540.htm Sulfuryl Fluoride, Pesticide Tolerance, Technical Correction; Federal Register; June 16, 2004 http://www.epa.gov/EPA-PEST/2004/June/Day-16/p13288.htm Sulfuryl Fluoride, Pesticide Tolerance; Federal Register; July 15, 2005 http://www.epa.gov/EPA-PEST/2005/July/Day-15/p13982.htm United States Prevention, Pesticides EPA-738-F-93-012 Environmental Protection And Toxic Substances September 1993 Agency (7508W) R.E.D. FACTS Sulfuryl Fluoride Pesticide All pesticides sold or distributed in the United States must be Reregistration registered by EPA, based on scientific studies showing that they can be used without posing unreasonable risks to people or the environment. Because of advances in scientific knowledge, the law requires that pesticides which were first registered years ago be reregistered to ensure that they meet today's more stringent standards. In evaluating pesticides for reregistration, EPA obtains and reviews a complete set of studies from pesticide producers, describing the human health and environmental effects of each pesticide. The Agency imposes any regulatory controls that are needed to effectively manage each pesticide's risks. -

PESTICIDES Criteria for a Recommended Standard
CRITERIA FOR A RECOMMENDED STANDARD OCCUPATIONAL EXPOSURE DURING THE MANUFACTURE AND FORMULATION OF PESTICIDES criteria for a recommended standard... OCCUPATIONAL EXPOSURE DURING THE MANUFACTURE AND FORMULATION OF PESTICIDES * U.S. DEPARTMENT OF HEALTH, EDUCATION, AND WELFARE Public Health Service Center for Disease Control National Institute for Occupational Safety and Health July 1978 For sale by the Superintendent of Documents, U.S. Government Printing Office, Washington, D.C. 20402 DISCLAIMER Mention of company names or products does not constitute endorsement by the National Institute for Occupational Safety and Health. DHEW (NIOSH) Publication No. 78-174 PREFACE The Occupational Safety and Health Act of 1970 emphasizes the need for standards to protect the health and provide for the safety of workers occupationally exposed to an ever-increasing number of potential hazards. The National Institute for Occupational Safety and Health (NIOSH) has implemented a formal system of research, with priorities determined on the basis of specified indices, to provide relevant data from which valid criteria for effective standards can be derived. Recommended standards for occupational exposure, which are the result of this work, are based on the effects of exposure on health. The Secretary of Labor will weigh these recommendations along with other considerations, such as feasibility and means of implementation, in developing regulatory standards. Successive reports will be presented as research and epideiriologic studies are completed and as sampling and analytical methods are developed. Criteria and standards will be reviewed periodically to ensure continuing protection of workers. The contributions to this document on pesticide manufacturing and formulating industries by NIOSH staff members, the review consultants, the reviewer selected by the American Conference of Governmental Industrial Hygienists (ACGIH), other Federal agencies, and by Robert B.