A High Volume Sampling System for Isotope Determination of Volatile Halocarbons and Hydrocarbons
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bromomethane CAS #: 74-83-9 Revised By: RRD Toxicology Unit Revision Date: August 14, 2015
CHEMICAL UPDATE WORKSHEET Chemical Name: Bromomethane CAS #: 74-83-9 Revised By: RRD Toxicology Unit Revision Date: August 14, 2015 (A) Chemical-Physical Properties Part 201 Value Updated Value Reference Source Comments Molecular Weight (g/mol) 94.94 94.94 EPI EXP Physical State at ambient temp Liquid Gas MDEQ Melting Point (˚C) 179 -93.70 EPI EXP Boiling Point (˚C) 3.5 3.50 EPI EXP Solubility (ug/L) 1.45E+7 1.52E+07 EPI EXP Vapor Pressure (mmHg at 25˚C) 1672 1.62E+03 EPI EXP HLC (atm-m³/mol at 25˚C) 1.42E-2 7.34E-03 EPI EXP Log Kow (log P; octanol-water) 1.18 1.19 EPI EXP Koc (organic carbon; L/Kg) 14.5 13.22 EPI EST Ionizing Koc (L/kg) NR NA NA Diffusivity in Air (Di; cm2/s) 0.08 1.00E-01 W9 EST Diffusivity in Water (Dw; cm2/s) 8.0E-6 1.3468E-05 W9 EST CHEMICAL UPDATE WORKSHEET Bromomethane (74-83-9) Part 201 Value Updated Value Reference Source Comments Soil Water Partition Coefficient NR NR NA NA (Kd; inorganics) Flash Point (˚C) NA 194 PC EXP Lower Explosivity Level (LEL; 0.1 0.1 CRC EXP unit less) Critical Temperature (K) 467.00 EPA2004 EXP Enthalpy of Vaporization 5.71E+03 EPA2004 EXP (cal/mol) Density (g/mL, g/cm3) 1.6755 CRC EXP EMSOFT Flux Residential 2 m 2.69E-05 2.80E-05 EMSOFT EST (mg/day/cm2) EMSOFT Flux Residential 5 m 6.53E-05 6.86E-05 EMSOFT EST (mg/day/cm2) EMSOFT Flux Nonresidential 2 m 3.83E-05 4.47E-05 EMSOFT EST (mg/day/cm2) EMSOFT Flux Nonresidential 5 m 9.24E-05 1.09E-04 EMSOFT EST (mg/day/cm2) 2 CHEMICAL UPDATE WORKSHEET Bromomethane (74-83-9) (B) Toxicity Values/Benchmarks Source/Reference/ Comments/Notes Part 201 Value Updated Value Date /Issues Reference Dose 1.4E-3 2.0E-2 OPP, 2013 (RfD) (mg/kg/day) Rat subchronic Tier 1 Source: Complete gavage study EPA-OPP: (Danse et al., Basis: OPP is the more current than IRIS, PPRTV and ATSDR. -

SAFETY DATA SHEET Bromomethane (R40 B1) SECTION 1
SAFETY DATA SHEET Bromomethane (R40 B1) Issue Date: 16.01.2013 Version: 1.0 SDS No.: 000010021848 Last revised date: 02.02.2017 1/17 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier Product name: Bromomethane (R40 B1) Additional identification Chemical name: Bromomethane Chemical formula: CH3Br INDEX No. 602-002-00-2 CAS-No. 74-83-9 EC No. 200-813-2 REACH Registration No. Not available. 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses: Industrial and professional. Perform risk assessment prior to use. Using gas alone or in mixtures for the calibration of analysis equipment. Using gas as feedstock in chemical processes. Formulation of mixtures with gas in pressure receptacles. Uses advised against Consumer use. 1.3 Details of the supplier of the safety data sheet Supplier Linde Gas GmbH Telephone: +43 50 4273 Carl-von-Linde-Platz 1 A-4651 Stadl-Paura E-mail: [email protected] 1.4 Emergency telephone number: Emergency number Linde: + 43 50 4273 (during business hours), Poisoning Information Center: +43 1 406 43 43 SDS_AT - 000010021848 SAFETY DATA SHEET Bromomethane (R40 B1) Issue Date: 16.01.2013 Version: 1.0 SDS No.: 000010021848 Last revised date: 02.02.2017 2/17 SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Directive 67/548/EEC or 1999/45/EC as amended. T; R23/25 Xi; R36/37/38 Xn; R48/20 Muta. 3; R68 N; R50 N; R59 The full text for all R-phrases is displayed in section 16. -

Iodomethane Safety Data Sheet 1100H01 According to Federal Register / Vol
Iodomethane Safety Data Sheet 1100H01 according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations Date of issue: 08/18/2016 Version: 1.0 SECTION 1: Identification 1.1. Identification Product form : Substance Substance name : Iodomethane CAS No : 74-88-4 Product code : 1100-H-01 Formula : CH3I Synonyms : Methyl iodide Other means of identification : MFCD00001073 1.2. Relevant identified uses of the substance or mixture and uses advised against Use of the substance/mixture : Laboratory chemicals Manufacture of substances Scientific research and development 1.3. Details of the supplier of the safety data sheet SynQuest Laboratories, Inc. P.O. Box 309 Alachua, FL 32615 - United States of America T (386) 462-0788 - F (386) 462-7097 [email protected] - www.synquestlabs.com 1.4. Emergency telephone number Emergency number : (844) 523-4086 (3E Company - Account 10069) SECTION 2: Hazard(s) identification 2.1. Classification of the substance or mixture Classification (GHS-US) Acute Tox. 3 (Oral) H301 - Toxic if swallowed Acute Tox. 3 (Dermal) H311 - Toxic in contact with skin Acute Tox. 2 (Inhalation) H330 - Fatal if inhaled Acute Tox. 3 (Inhalation:vapour) H331 - Toxic if inhaled Skin Irrit. 2 H315 - Causes skin irritation Eye Dam. 1 H318 - Causes serious eye damage Resp. Sens. 1 H334 - May cause allergy or asthma symptoms or breathing difficulties if inhaled Skin Sens. 1 H317 - May cause an allergic skin reaction Carc. 2 H351 - Suspected of causing cancer STOT SE 3 H335 - May cause respiratory irritation -

METHYL IODIDE 1. Exposure Data
METHYL IODIDE Data were last reviewed in IARC (1986) and the compound was classified in IARC Monographs Supplement 7 (1987). 1. Exposure Data 1.1 Chemical and physical data 1.1.1 Nomenclature Chem. Abstr. Serv. Reg. No.: 74-88-4 Chem. Abstr. Name: Iodomethane IUPAC Systematic Name: Iodomethane 1.1.2 Structural and molecular formulae and relative molecular mass H H C I H CH3I Relative molecular mass: 141.94 1.1.3 Chemical and physical properties of the pure substance (a) Description: Colourless transparent liquid, with a sweet ethereal odour (American Conference of Governmental Industrial Hygienists, 1992; Budavari, 1996) (b) Boiling-point: 42.5°C (Lide, 1997) (c) Melting-point: –66.4°C (Lide, 1997) (d) Solubility: Slightly soluble in water (14 g/L at 20°C); soluble in acetone; miscible with diethyl ether and ethanol (Budavari, 1996; Verschueren, 1996; Lide, 1997) (e) Vapour pressure: 53 kPa at 25.3°C; relative vapour density (air = 1), 4.9 (Ver- schueren, 1996) ( f ) Octanol/water partition coefficient (P): log P, 1.51 (Hansch et al., 1995) (g) Conversion factor: mg/m3 = 5.81 × ppm –1503– 1504 IARC MONOGRAPHS VOLUME 71 1.2 Production and use No information on the global production of methyl iodide was available to the Working Group. Production in the United States in 1983 was about 50 tonnes (IARC, 1986). Because of its high refractive index, methyl iodide is used in microscopy. It is also used as an embedding material for examining diatoms, in testing for pyridine, as a methy- lating agent in pharmaceutical (e.g., quaternary ammonium compounds) and chemical synthesis, as a light-sensitive etching agent for electronic circuits, and as a component in fire extinguishers (IARC, 1986; American Conference of Governmental Industrial Hygienists, 1992; Budavari, 1996). -

Aldrich Organometallic, Inorganic, Silanes, Boranes, and Deuterated Compounds
Aldrich Organometallic, Inorganic, Silanes, Boranes, and Deuterated Compounds Library Listing – 1,523 spectra Subset of Aldrich FT-IR Library related to organometallic, inorganic, boron and deueterium compounds. The Aldrich Material-Specific FT-IR Library collection represents a wide variety of the Aldrich Handbook of Fine Chemicals' most common chemicals divided by similar functional groups. These spectra were assembled from the Aldrich Collections of FT-IR Spectra Editions I or II, and the data has been carefully examined and processed by Thermo Fisher Scientific. Aldrich Organometallic, Inorganic, Silanes, Boranes, and Deuterated Compounds Index Compound Name Index Compound Name 1066 ((R)-(+)-2,2'- 1193 (1,2- BIS(DIPHENYLPHOSPHINO)-1,1'- BIS(DIPHENYLPHOSPHINO)ETHAN BINAPH)(1,5-CYCLOOCTADIENE) E)TUNGSTEN TETRACARBONYL, 1068 ((R)-(+)-2,2'- 97% BIS(DIPHENYLPHOSPHINO)-1,1'- 1062 (1,3- BINAPHTHYL)PALLADIUM(II) CH BIS(DIPHENYLPHOSPHINO)PROPA 1067 ((S)-(-)-2,2'- NE)DICHLORONICKEL(II) BIS(DIPHENYLPHOSPHINO)-1,1'- 598 (1,3-DIOXAN-2- BINAPH)(1,5-CYCLOOCTADIENE) YLETHYNYL)TRIMETHYLSILANE, 1140 (+)-(S)-1-((R)-2- 96% (DIPHENYLPHOSPHINO)FERROCE 1063 (1,4- NYL)ETHYL METHYL ETHER, 98 BIS(DIPHENYLPHOSPHINO)BUTAN 1146 (+)-(S)-N,N-DIMETHYL-1-((R)-1',2- E)(1,5- BIS(DI- CYCLOOCTADIENE)RHODIUM(I) PHENYLPHOSPHINO)FERROCENY TET L)E 951 (1,5-CYCLOOCTADIENE)(2,4- 1142 (+)-(S)-N,N-DIMETHYL-1-((R)-2- PENTANEDIONATO)RHODIUM(I), (DIPHENYLPHOSPHINO)FERROCE 99% NYL)ETHYLAMIN 1033 (1,5- 407 (+)-3',5'-O-(1,1,3,3- CYCLOOCTADIENE)BIS(METHYLD TETRAISOPROPYL-1,3- IPHENYLPHOSPHINE)IRIDIUM(I) -

RR Program's RCL Spreadsheet Update
RR Program’s RCL Spreadsheet Update March 2017 RR Program RCL Spreadsheet Update DNR-RR-052e The Wisconsin DNR Remediation and Redevelopment Program (RR) has updated the numerical soil standards in the August 2015 DNR-RR- 052b RR spreadsheet of residual contaminant levels (RCLs). The RCLs were determined using the U.S. EPA RSL web- calculator by accepting EPA exposure defaults, with the exception of using Chicago, IL, for the climatic zone. This documentThe U.S. provides EPA updateda summary its Regionalof changes Screening to the direct-contact Level (RSL) RCLs website (DC-RCLs) in June that2015. are To now reflect in the that March 2017 spreadsheet.update, the The Wisconsin last page ofDNR this updated document the has numerical the EPA exposuresoil standards, parameter or residual values usedcontaminant in the RCL levels calculations. (RCLs), in the Remediation and Redevelopment program’s spreadsheet of RCLs. This document The providesU.S. EPA a RSL summary web-calculator of the updates has been incorporated recently updated in the Julyso that 2015 the spreadsheet.most up-to-date There toxicity were values no changes for chemi - cals madewere certainlyto the groundwater used in the RCLs,RCL calculations. but there are However, many changes it is important in the industrial to note that and the non-industrial web-calculator direct is only a subpartcontact of the (DC) full RCLsEPA RSL worksheets. webpage, Tables and that 1 andthe other 2 of thissubparts document that will summarize have important the DC-RCL explanatory changes text, generic tablesfrom and the references previous have spreadsheet yet to be (Januaryupdated. -
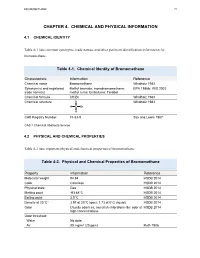
Toxicological Profile for Bromomethane
BROMOMETHANE 72 CHAPTER 4. CHEMICAL AND PHYSICAL INFORMATION 4.1 CHEMICAL IDENTITY Table 4-1 lists common synonyms, trade names, and other pertinent identification information for bromomethane. Table 4-1. Chemical Identity of Bromomethane Characteristic Information Reference Chemical name Bromomethane Windholz 1983 Synonym(s) and registered Methyl bromide; monobromomethane; EPA 1986b; IRIS 2002 trade name(s) methyl fume; Embafume; Terabol Chemical formula CH3Br Windholz 1983 Chemical structure H Windholz 1983 H C Br H CAS Registry Number 74-83-9 Sax and Lewis 1987 CAS = Chemical Abstracts Service 4.2 PHYSICAL AND CHEMICAL PROPERTIES Table 4-2 lists important physical and chemical properties of bromomethane. Table 4-2. Physical and Chemical Properties of Bromomethane Property Information Reference Molecular weight 94.94 HSDB 2014 Color Colorless HSDB 2014 Physical state Gas HSDB 2014 Melting point -93.68°C HSDB 2014 Boiling point 3.5°C HSDB 2014 Density at 20°Ca 3.97 at 20°C (gas); 1.73 at 0°C (liquid) HSDB 2014 Odor Usually odorless; sweetish chloroform-like odor at HSDB 2014 high concentrations Odor threshold: Water No data Air 80 mg/m3 (20 ppm) Ruth 1986 BROMOMETHANE 73 4. CHEMICAL AND PHYSICAL INFORMATION Table 4-2. Physical and Chemical Properties of Bromomethane Solubility: Water at 20°C 15.2 g/L at 25°C HSDB 2014 18.5 g/L at 20°C 13.4 g/L at 25°C Organic solvents Readily soluble in lower alcohols, ethers, esters, HSDB 2014 ketones, halogenated hydrocarbons, aromatic hydrocarbons, and carbon disulfide; freely soluble in benzene, carbon tetrachloride, and carbon disulfide; miscible in ethanol and chloroform Partition coefficients: Log Kow 1.19 HSDB 2014 Log Koc 0.95–1.3 Yates et al. -

Methyl Iodide (Iodomethane)
Methyl Iodide (Iodomethane) 74-88-4 Hazard Summary Methyl iodide is used as an intermediate in the manufacture of some pharmaceuticals and pesticides, in methylation processes, and in the field of microscopy. In humans, acute (short-term) exposure to methyl iodide by inhalation may depress the central nervous system (CNS), irritate the lungs and skin, and affect the kidneys. Massive acute inhalation exposure to methyl iodide has led to pulmonary edema. Acute inhalation exposure of humans to methyl iodide has resulted in nausea, vomiting, vertigo, ataxia, slurred speech, drowsiness, skin blistering, and eye irritation. Chronic (long-term) exposure of humans to methyl iodide by inhalation may affect the CNS and cause skin burns. EPA has not classified methyl iodide for potential carcinogenicity. Please Note: The main sources of information for this fact sheet are the International Agency for Research on Cancer (IARC) monographs on chemicals carcinogenic to humans (2) and the Hazardous Substances Data Bank (HSDB) (3), a database of summaries of peer-reviewed literature. Uses Methyl iodide is used as an intermediate in the manufacture of some pharmaceuticals and pesticides. It is also used in methylation processes and in the field of microscopy. (1,2,4) Proposed uses of methyl iodide are as a fire extinguisher and as an insecticidal fumigant. (5) Sources and Potential Exposure Individuals are most likely to be exposed to methyl iodide in the workplace. (1) Methyl iodide occurs naturally in the ocean as a product of marine algae. (2) Assessing Personal Exposure No information was located regarding the measurement of personal exposure to methyl iodide. -

Measurement of Formic and Acetic Acid in Air by Chemical Ionization Mass Spectroscopy: Airborne Method Development
University of Rhode Island DigitalCommons@URI Open Access Master's Theses 2015 Measurement of Formic and Acetic Acid in Air by Chemical Ionization Mass Spectroscopy: Airborne Method Development Victoria Treadaway University of Rhode Island, [email protected] Follow this and additional works at: https://digitalcommons.uri.edu/theses Recommended Citation Treadaway, Victoria, "Measurement of Formic and Acetic Acid in Air by Chemical Ionization Mass Spectroscopy: Airborne Method Development" (2015). Open Access Master's Theses. Paper 603. https://digitalcommons.uri.edu/theses/603 This Thesis is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Master's Theses by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. MEASUREMENT OF FORMIC AND ACETIC ACID IN AIR BY CHEMICAL IONIZATION MASS SPECTROSCOPY: AIRBORNE METHOD DEVELOPMENT BY VICTORIA TREADAWAY A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN OCEANOGRAPHY UNIVERSITY OF RHODE ISLAND 2015 MASTER OF SCIENCE THESIS OF VICTORIA TREADAWAY APPROVED: Thesis Committee: Major Professor Brian Heikes John Merrill James Smith Nasser H. Zawia DEAN OF THE GRADUATE SCHOOL UNIVERSITY OF RHODE ISLAND 2015 ABSTRACT The goals of this study were to determine whether formic and acetic acid could be quantified from the Deep Convective Clouds and Chemistry Experiment (DC3) through post-mission calibration and analysis and to optimize a reagent gas mix with CH3I, CO2, and O2 that allows quantitative cluster ion formation with hydroperoxides and organic acids suitable for use in future field measurements. -

Gas Phase Chemistry and Removal of CH3I During a Severe Accident
DK0100070 Nordisk kernesikkerhedsforskning Norraenar kjarnoryggisrannsoknir Pohjoismainenydinturvallisuustutkimus Nordiskkjernesikkerhetsforskning Nordisk karnsakerhetsforskning Nordic nuclear safety research NKS-25 ISBN 87-7893-076-6 Gas Phase Chemistry and Removal of CH I during a Severe Accident Anna Karhu VTT Energy, Finland 2/42 January 2001 Abstract The purpose of this literature review was to gather valuable information on the behavior of methyl iodide on the gas phase during a severe accident. The po- tential of transition metals, especially silver and copper, to remove organic io- dides from the gas streams was also studied. Transition metals are one of the most interesting groups in the contex of iodine mitigation. For example silver is known to react intensively with iodine compounds. Silver is also relatively inert material and it is thermally stable. Copper is known to react with some radioio- dine species. However, it is not reactive toward methyl iodide. In addition, it is oxidized to copper oxide under atmospheric conditions. This may limit the in- dustrial use of copper. Key words Methyl iodide, gas phase, severe accident mitigation NKS-25 ISBN 87-7893-076-6 Danka Services International, DSI, 2001 The report can be obtained from NKS Secretariat P.O. Box 30 DK-4000RoskiIde Denmark Phone +45 4677 4045 Fax +45 4677 4046 http://www.nks.org e-mail: [email protected] Gas Phase Chemistry and Removal of CH3I during a Severe Accident VTT Energy, Finland Anna Karhu Abstract The purpose of this literature review was to gather valuable information on the behavior of methyl iodide on the gas phase during a severe accident. -

Federal Register/Vol. 84, No. 230/Friday, November 29, 2019
Federal Register / Vol. 84, No. 230 / Friday, November 29, 2019 / Proposed Rules 65739 are operated by a government LIBRARY OF CONGRESS 49966 (Sept. 24, 2019). The Office overseeing a population below 50,000. solicited public comments on a broad Of the impacts we estimate accruing U.S. Copyright Office range of subjects concerning the to grantees or eligible entities, all are administration of the new blanket voluntary and related mostly to an 37 CFR Part 210 compulsory license for digital uses of increase in the number of applications [Docket No. 2019–5] musical works that was created by the prepared and submitted annually for MMA, including regulations regarding competitive grant competitions. Music Modernization Act Implementing notices of license, notices of nonblanket Therefore, we do not believe that the Regulations for the Blanket License for activity, usage reports and adjustments, proposed priorities would significantly Digital Uses and Mechanical Licensing information to be included in the impact small entities beyond the Collective: Extension of Comment mechanical licensing collective’s potential for increasing the likelihood of Period database, database usability, their applying for, and receiving, interoperability, and usage restrictions, competitive grants from the Department. AGENCY: U.S. Copyright Office, Library and the handling of confidential of Congress. information. Paperwork Reduction Act ACTION: Notification of inquiry; To ensure that members of the public The proposed priorities do not extension of comment period. have sufficient time to respond, and to contain any information collection ensure that the Office has the benefit of SUMMARY: The U.S. Copyright Office is requirements. a complete record, the Office is extending the deadline for the extending the deadline for the Intergovernmental Review: This submission of written reply comments program is subject to Executive Order submission of written reply comments in response to its September 24, 2019 to no later than 5:00 p.m. -

Provisional Peer-Reviewed Toxicity Values for Iodomethane (Methyl
FINAL 9-30-2009 Provisional Peer-Reviewed Toxicity Values for Iodomethane (Methyl Iodide) (CASRN 74-88-4) Superfund Health Risk Technical Support Center National Center for Environmental Assessment Office of Research and Development U.S. Environmental Protection Agency Cincinnati, OH 45268 COMMONLY USED ABBREVIATIONS BMD Benchmark Dose IRIS Integrated Risk Information System IUR inhalation unit risk LOAEL lowest-observed-adverse-effect level LOAELADJ LOAEL adjusted to continuous exposure duration LOAELHEC LOAEL adjusted for dosimetric differences across species to a human NOAEL no-observed-adverse-effect level NOAELADJ NOAEL adjusted to continuous exposure duration NOAELHEC NOAEL adjusted for dosimetric differences across species to a human NOEL no-observed-effect level OSF oral slope factor p-IUR provisional inhalation unit risk p-OSF provisional oral slope factor p-RfC provisional inhalation reference concentration p-RfD provisional oral reference dose RfC inhalation reference concentration RfD oral reference dose UF uncertainty factor UFA animal to human uncertainty factor UFC composite uncertainty factor UFD incomplete to complete database uncertainty factor UFH interhuman uncertainty factor UFL LOAEL to NOAEL uncertainty factor UFS subchronic to chronic uncertainty factor i FINAL 9-30-2009 PROVISIONAL PEER-REVIEWED TOXICITY VALUES FOR IODOMETHANE (CASRN 74-88-4) Background On December 5, 2003, the U.S. Environmental Protection Agency’s (U.S. EPA) Office of Superfund Remediation and Technology Innovation (OSRTI) revised its hierarchy of human health toxicity values for Superfund risk assessments, establishing the following three tiers as the new hierarchy: 1) U.S. EPA’s Integrated Risk Information System (IRIS). 2) Provisional Peer-Reviewed Toxicity Values (PPRTVs) used in U.S.