Alterations in Vascular Function and Morphology in Acute Ischemic Renal Failure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Kidney, Renal Tubule – Dilation
Kidney, Renal Tubule – Dilation Figure Legend: Figure 1 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Dilated tubules are noted as tracts running through the cortex and outer medulla. Figure 2 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is present throughout the outer stripe of the outer medulla, extending into the cortex. Figure 3 Kidney, Renal tubule - Dilation in a male B6C3F1 mouse from a chronic study. Slight tubule dilation is associated with degeneration and necrosis. Figure 4 Kidney, Renal tubule - Dilation in a male F344/N rat from a chronic study. Tubule dilation is associated with chronic progressive nephropathy. Comment: Renal tubule dilation may occur anywhere along the nephron or collecting duct system. It may occur in focal areas or as tracts running along the entire length of kidney sections (Figure 1). 1 Kidney, Renal Tubule – Dilation Renal tubule dilation may occur from xenobiotic administration, secondary mechanisms, or an unknown pathogenesis (see Kidney – Nephropathy, Obstructive (Figure 2). Dilation may result from direct toxic injury to the tubule epithelium interfering with absorption and secretion (Figure 3). It may also occur secondary to renal ischemia or from prolonged diuresis related to drug administration. Secondary mechanisms of tubule dilation may result from lower urinary tract obstruction, the deposition of tubule crystals, interstitial inflammation and/or fibrosis, and chronic progressive nephropathy (Figure 4). A few dilated tubules may be regarded as normal histologic variation. Recommendation: Renal tubule dilation should be diagnosed and given a severity grade. The location of tubule dilation should be included in the diagnosis as a site modifier. -
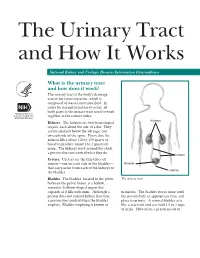
The Urinary Tract and How It Works
The Urinary Tract and How It Works National Kidney and Urologic Diseases Information Clearinghouse What is the urinary tract and how does it work? The urinary tract is the body’s drainage system for removing urine, which is composed of wastes and extra fluid. In order for normal urination to occur, all body parts in the urinary tract need to work together in the correct order. Kidneys Kidneys. The kidneys are two bean-shaped organs, each about the size of a fist. They are located just below the rib cage, one on each side of the spine. Every day, the kidneys filter about 120 to 150 quarts of blood to produce about 1 to 2 quarts of urine. The kidneys work around the clock; a person does not control what they do. Ureters Ureters. Ureters are the thin tubes of muscle—one on each side of the bladder— Bladder that carry urine from each of the kidneys to Urethra the bladder. Bladder. The bladder, located in the pelvis The urinary tract between the pelvic bones, is a hollow, muscular, balloon-shaped organ that expands as it fills with urine. Although a urination. The bladder stores urine until person does not control kidney function, the person finds an appropriate time and a person does control when the bladder place to urinate. A normal bladder acts empties. Bladder emptying is known as like a reservoir and can hold 1.5 to 2 cups of urine. How often a person needs to urinate depends on how quickly the kidneys Why is the urinary tract produce the urine that fills the bladder. -

Claudins in the Renal Collecting Duct
International Journal of Molecular Sciences Review Claudins in the Renal Collecting Duct Janna Leiz 1,2 and Kai M. Schmidt-Ott 1,2,3,* 1 Department of Nephrology and Intensive Care Medicine, Charité-Universitätsmedizin Berlin, 12203 Berlin, Germany; [email protected] 2 Molecular and Translational Kidney Research, Max-Delbrück-Center for Molecular Medicine in the Helmholtz Association (MDC), 13125 Berlin, Germany 3 Berlin Institute of Health (BIH), 10178 Berlin, Germany * Correspondence: [email protected]; Tel.: +49-(0)30-450614671 Received: 22 October 2019; Accepted: 20 December 2019; Published: 28 December 2019 Abstract: The renal collecting duct fine-tunes urinary composition, and thereby, coordinates key physiological processes, such as volume/blood pressure regulation, electrolyte-free water reabsorption, and acid-base homeostasis. The collecting duct epithelium is comprised of a tight epithelial barrier resulting in a strict separation of intraluminal urine and the interstitium. Tight junctions are key players in enforcing this barrier and in regulating paracellular transport of solutes across the epithelium. The features of tight junctions across different epithelia are strongly determined by their molecular composition. Claudins are particularly important structural components of tight junctions because they confer barrier and transport properties. In the collecting duct, a specific set of claudins (Cldn-3, Cldn-4, Cldn-7, Cldn-8) is expressed, and each of these claudins has been implicated in mediating aspects of the specific properties of its tight junction. The functional disruption of individual claudins or of the overall barrier function results in defects of blood pressure and water homeostasis. In this concise review, we provide an overview of the current knowledge on the role of the collecting duct epithelial barrier and of claudins in collecting duct function and pathophysiology. -

Congenital Anomalies of Kidney and Ureter
ogy: iol Cu ys r h re P n t & R y e s Anatomy & Physiology: Current m e o a t Mittal et al., Anat Physiol 2016, 6:1 r a c n h A Research DOI: 10.4172/2161-0940.1000190 ISSN: 2161-0940 Review Article Open Access Congenital Anomalies of Kidney and Ureter Mittal MK1, Sureka B1, Mittal A2, Sinha M1, Thukral BB1 and Mehta V3* 1Department of Radiodiagnosis, Safdarjung Hospital, India 2Department of Paediatrics, Safdarjung Hospital, India 3Department of Anatomy, Safdarjung Hospital, India Abstract The kidney is a common site for congenital anomalies which may be responsible for considerable morbidity among young patients. Radiological investigations play a central role in diagnosing these anomalies with the screening ultrasonography being commonly used as a preliminary diagnostic study. Intravenous urography can be used to specifically identify an area of obstruction and to determine the presence of duplex collecting systems and a ureterocele. Computed tomography and magnetic resonance (MR) imaging are unsuitable for general screening but provide superb anatomic detail and added diagnostic specificity. A sound knowledge of the anatomical details and familiarity with these anomalies is essential for correct diagnosis and appropriate management so as to avoid the high rate of morbidity associated with these malformations. Keywords: Kidney; Ureter; Intravenous urography; Duplex a separate ureter is seen then the supernumerary kidney is located cranially in relation to the normal kidney. In such a case the ureter Introduction enters the bladder ectopically and according to the Weigert-R Meyer Congenital anomalies of the kidney and ureter are a significant cause rule the ureter may insert medially and inferiorly into the bladder [2]. -
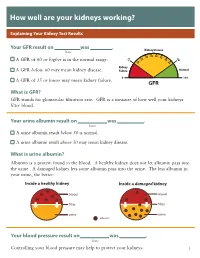
How Well Are Your Kidneys Working?
How well are your kidneys working? Explaining Your Kidney Test Results Kidney Your GFR result on was . Kidney Disease Date 35 40 0 45 3 6 5 5 50 0 A GFR of 60 or higher is in the normal range. 1 2 5 0 5 2 Kidney A GFR below 60 may mean kidney disease. Failure Normal 0 120 A GFR of 15 or lower may mean kidney failure. GFR What is GFR? GFR stands for glomerular filtration rate. GFR is a measure of how well your kidneys filter blood. Your urine albumin result on was . Date A urine albumin result below 30 is normal. A urine albumin result above 30 may mean kidney disease. What is urine albumin? Albumin is a protein found in the blood. A healthy kidney does not let albumin pass into the urine. A damaged kidney lets some albumin pass into the urine. The less albumin in your urine, the better. Inside a healthy kidney Inside a damaged kidney blood blood filter filter urine urine albumin Your blood pressure result on was . Date Controlling your blood pressure may help to protect your kidneys. 1 What your kidneys do You have two kidneys. Their main job is to filter wastes and extra water out of your blood to make urine. kidneys How your kidneys are checked Two tests are used to check for kidney disease. • A blood test checks your GFR, which tells how well your kidneys are filtering. • A urine test checks for albumin in your urine, a sign of kidney damage. Why your kidneys are being checked You need to have your kidneys checked because you can’t feel kidney disease. -

Hydronephrosis
Patient and Family Education Hydronephrosis What is Hydronephrosis? Hydronephrosis is a dilation of the kidney, specifically in the renal pelvis or the place in the kidney where urine is stored after its production. It occurs in 1-2% of all pregnancies. This extra fluid can be the result of some type of abnormality in or below the kidney or it may be a variant of normal. Hydronephrosis can be caused by many factors. Some of the most common reasons include obstruction or urinary reflux. Obstruction of the kidneys can occur at the level of the kidney (uretero-pelvic junction obstruction or UPJ) or at the level of the bladder (uretero-vesical junction or UVJ). It can also include a megaureter. Urinary reflux is the abnormal back flow of urine from the bladder back towards the kidneys. Obstruction: Reflux: How is hydronephrosis diagnosed? Hydronephrosis is usually diagnosed in one of two ways. 1. A prenatal ultrasound (ultrasound during pregnancy). This may reveal that the unborn baby has dilated or enlarged kidneys. This occurs in about 1 out of 100 pregnancies. 2. An ultrasound done after the baby is born. This sometimes is found after a routine evaluation for another medical problem or concern such as urinary tract infection or incontinence. Once hydronephrosis is noted, additional tests may be needed in order to find out why there is extra fluid in the kidneys. Early diagnosis and treatment of such an abnormality can prevent future urinary tract infections and permanent kidney damage or scarring. How is hydronephrosis graded and why it this important? Hydronephrosis is graded on a scale ranging from 1-4, with one being the mildest form and four being the most severe. -

HAVING a URETERIC STENT What to Expect and How to Manage
You have been sent home with HAVING A URETERIC STENT What to expect and how to manage 1 INTRODUCTION In patients who have, or might have an obstruction (blockage) of the kidney, an internal drainage tube called a “stent” is commonly placed in the ureter, the tube between the kidney and the bladder. This is placed there in order to prevent of temporarily relieve the obstruction. This information sheet has been given to you to help answer some of the questions you may have about having a ureteric stent inserted. If you have any questions or concerns, please, do not hesitate to speak to a doctor or a nurse caring for you. 2 THE URINARY SYSTEM AND URETERIC STENT THE URINARY SYSTEM AND THE URETER The kidneys produce urine. Normally there are two kidneys situated in the upper part of the abdomen, towards the back. The urine formed in the kidney is carried to the bladder by a fine muscular tube called a ureter. The urinary bladder acts as a reservoir for the urine and when it is full it is emptied via the urethra. Figure 1: The urinary system HOW DOES A KIDNEY BECOME OBSTRUCTED ? Common causes of obstruction of the kidneys and ureter are: -A kidney stone or its fragments moving into the ureter, either spontaneously, or occasionally following such treatment as shock wave therapy. -Narrrowing (stricture) of the ureter anywhere along its path. This can be due. scaring of wall of the ureter, narrowing of the area where the ureter leaves from the kidney (pelvi-ueteric junction) 3 -Temporarily, following an operation or after an instrument has been inserted into the ureter and kidneys. -

Choosing a Treatment for Kidney Failure
CHOOSING A TREATMENT FOR KIDNEY FAILURE www.kidney.org National Kidney Foundation's Kidney Disease Outcomes Quality Initiative Did you know that the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (NKF-KDOQI™) offers guidelines and commentaries that help your doctor and healthcare team make important decisions about your medical treatment? The information in this booklet is based on those recommended guidelines. What is your stage of kidney disease? There are 5 stages of kidney disease. They are shown in the table below. Your doctor determines your stage of kidney disease based on the presence of kidney damage and your glomerular filtration rate (GFR), which is a measure of your level of kidney function. Your treatment is based on your stage of kidney disease. Speak to your doctor if you have any questions about your stage of kidney disease or your treatment. STAGES OF KIDNEY DISEASE Stage Description Glomerular Filtration Rate (GFR)* Kidney damage (e.g., protein 1 90 or above in the urine) with normal GFR Kidney damage with mild 2 60 to 89 decrease in GFR 3 Moderate decrease in GFR 30 to 59 4 Severe reduction in GFR 15 to 29 5 Kidney failure Less than 15 *Your GFR number tells your doctor how much kidney function you have. As chronic kidney disease progresses, your GFR number decreases. TABLE OF CONTENTS Kidney Failure ............................... 5 Hemodialysis ............................... 8 Peritoneal Dialysis ...........................14 Kidney Transplant ...........................16 Living with Your Treatment Choice .............17 For More Information. 24 If your doctor said you may need dialysis or a kidney transplant soon, you are probably wondering how to decide on the best choice for you. -

Urine-Derived Epithelial Cells As Models for Genetic Kidney Diseases
cells Review Urine-Derived Epithelial Cells as Models for Genetic Kidney Diseases Tjessa Bondue 1 , Fanny O. Arcolino 1 , Koenraad R. P. Veys 1,2, Oyindamola C. Adebayo 1,3, Elena Levtchenko 1,2, Lambertus P. van den Heuvel 1,4 and Mohamed A. Elmonem 5,* 1 Department of Development and Regeneration, KU Leuven, 3000 Leuven, Belgium; [email protected] (T.B.); [email protected] (F.O.A.); [email protected] (K.R.P.V.); [email protected] (O.C.A.); [email protected] (E.L.); [email protected] (L.P.v.d.H.) 2 Department of Pediatrics, Division of Pediatric Nephrology, University Hospitals Leuven, 3000 Leuven, Belgium 3 Centre for Molecular and Vascular Biology, Department of Cardiovascular Sciences, KU Leuven, 3000 Leuven, Belgium 4 Department of Pediatric Nephrology, Radboud University Medical Center, 6500 Nijmegen, The Netherlands 5 Department of Clinical and Chemical Pathology, Faculty of Medicine, Cairo University, Cairo 11628, Egypt * Correspondence: [email protected] Abstract: Epithelial cells exfoliated in human urine can include cells anywhere from the urinary tract and kidneys; however, podocytes and proximal tubular epithelial cells (PTECs) are by far the most relevant cell types for the study of genetic kidney diseases. When maintained in vitro, they have been proven extremely valuable for discovering disease mechanisms and for the development of new therapies. Furthermore, cultured patient cells can individually represent their human sources and their specific variants for personalized medicine studies, which are recently gaining much Citation: Bondue, T.; Arcolino, F.O.; interest. In this review, we summarize the methodology for establishing human podocyte and PTEC Veys, K.R.P.; Adebayo, O.C.; cell lines from urine and highlight their importance as kidney disease cell models. -

Preserving Kidney Function When You Have Diabetes Steps to Prevent Kidney Damage Or Halt Its Progress
Diabetes Education – #26 Preserving Kidney Function When You Have Diabetes Steps to prevent kidney damage or halt its progress Over time, diabetes can harm the kidneys. This can lead to kidney failure. But here’s the good news. Many people with diabetes do not end up with kidney problems. You can help prevent kidney damage or keep it from getting worse. When kidney function goes wrong The kidneys filter waste products and excess fluid from the blood. Blood goes into the kidney through an artery. It then passes through clusters of small blood vessels. The clusters (“glomeruli”) act as filters. The cleaned blood returns to the rest of the body through a vein. Waste products and excess fluid go into the urine. High blood sugar and high blood pressure can harm the filters. This may let protein leak into the urine. Kidney damage may begin 10 to 15 years after diabetes starts. As damage gets worse, the kidneys become worse at cleansing the blood. If the damage gets bad enough, the kidneys can stop working. Kidney damage can’t be reversed. And you may not feel it until there’s a lot of damage. So, take steps now to prevent kidney damage or stop its progress. Assessing your risk Some ethnic groups are at greater risk for kidney damage from diabetes. These include: • Native Americans • Hispanic people • African-Americans People in these groups should take extra care to protect their kidneys. Diabetes Education – #26 Do you have a parent or sibling with kidney damage from diabetes? If so, this raises your risk. -

Ureteric Stent
Ureteric stent Information for patients What is a ureteric stent? A ureteric stent is a thin plastic tube which is inserted into the ureter between your kidney and your bladder. This allows the urine produced by your kidney to pass easily into your bladder. kidney stent ureter bladder opening of the ureter page 2 Why do I have a ureteric stent? You have had a ureteric stent inserted because of an obstruction (blockage) in your ureter. The stent makes a channel, allowing the urine to flow from the kidney, helping the kidney to drain. Common causes of obstruction are: • a kidney stone or parts of a stone that have broken away • narrowing of the ureter anywhere along its path • from inflammation following an operation, or after an instrument has been inserted into the ureter and kidney. Are there any side effects? You should be able to carry on with your usual life with a ureteric stent in place, but you are likely to be able to feel its presence most of the time. Side effects are usually quite mild, but there is a risk that they may become severe. Side effects include: • an increased frequency in passing urine (needing to go more often) • the need to rush to the toilet to pass urine (urgency) • blood in the urine. This is quite common and you should drink around 2 litres (3 and a half pints) of water each day, to help flush your system through. • a sensation that your bladder is not completely empty after passing urine • a slight risk of episodes of incontinence (leaking of urine). -
YOUR KIDNEYS: Master Chemists of the Body
YOUR KIDNEYS: Master Chemists of the Body www.kidney.org National Kidney Foundation's Kidney Disease Outcomes Quality Initiative Did you know that the National Kidney Foundation's Kidney Disease Outcomes Quality Initiative (NKF-KDOQI™) offers guidelines and commentaries that help your doctor and healthcare team make important decisions about your medical treatment? The information in this booklet is based on those recommended guidelines. Stages of Kidney Disease There are 5 stages of kidney disease. They are shown in the table below. Your doctor determines your stage of kidney disease based on the presence of kidney damage and your glomerular filtration rate (GFR), which is a measure of your level of kidney function. Your treatment is based on your stage of kidney disease. Speak to your doctor if you have any questions about your stage of kidney disease or your treatment. STAGES OF KIDNEY DISEASE Glomerular Stage Description Filtration Rate (GFR)* Kidney damage (e.g., protein 1 90 or above in the urine) with normal GFR Kidney damage with mild 2 60 to 89 decrease in GFR 3 Moderate decrease in GFR 30 to 59 4 Severe reduction in GFR 15 to 29 5 Kidney failure Less than 15 *Your GFR number tells your doctor how much kidney function you have. As chronic kidney disease progresses, your GFR number decreases. TABLE OF CONTENTS Why are the kidneys so important? ..................5 Where are the kidneys and how do they function? .......6 What is chronic kidney disease? ... 7 What are some of the causes of kidney disease? ..............8 How is kidney disease detected? ..12 What are the warning signs of kidney disease? ............