The Pennsylvania State University
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Indiana Species April 2007
Fishes of Indiana April 2007 The Wildlife Diversity Section (WDS) is responsible for the conservation and management of over 750 species of nongame and endangered wildlife. The list of Indiana's species was compiled by WDS biologists based on accepted taxonomic standards. The list will be periodically reviewed and updated. References used for scientific names are included at the bottom of this list. ORDER FAMILY GENUS SPECIES COMMON NAME STATUS* CLASS CEPHALASPIDOMORPHI Petromyzontiformes Petromyzontidae Ichthyomyzon bdellium Ohio lamprey lampreys Ichthyomyzon castaneus chestnut lamprey Ichthyomyzon fossor northern brook lamprey SE Ichthyomyzon unicuspis silver lamprey Lampetra aepyptera least brook lamprey Lampetra appendix American brook lamprey Petromyzon marinus sea lamprey X CLASS ACTINOPTERYGII Acipenseriformes Acipenseridae Acipenser fulvescens lake sturgeon SE sturgeons Scaphirhynchus platorynchus shovelnose sturgeon Polyodontidae Polyodon spathula paddlefish paddlefishes Lepisosteiformes Lepisosteidae Lepisosteus oculatus spotted gar gars Lepisosteus osseus longnose gar Lepisosteus platostomus shortnose gar Amiiformes Amiidae Amia calva bowfin bowfins Hiodonotiformes Hiodontidae Hiodon alosoides goldeye mooneyes Hiodon tergisus mooneye Anguilliformes Anguillidae Anguilla rostrata American eel freshwater eels Clupeiformes Clupeidae Alosa chrysochloris skipjack herring herrings Alosa pseudoharengus alewife X Dorosoma cepedianum gizzard shad Dorosoma petenense threadfin shad Cypriniformes Cyprinidae Campostoma anomalum central stoneroller -

Melanoides Tuberculata), Species Habitat Associations and Life History Investigations in the San Solomon Spring Complex, Texas
FINAL REPORT As Required by THE ENDANGERED SPECIES PROGRAM TEXAS Grant No. TX E-121-R Endangered and Threatened Species Conservation Native springsnails and the invasive red-rim melania snail (Melanoides tuberculata), species habitat associations and life history investigations in the San Solomon Spring complex, Texas Prepared by: David Rogowski Carter Smith Executive Director Clayton Wolf Director, Wildlife 3 October 2012 FINAL REPORT STATE: ____Texas_______________ GRANT NUMBER: ___ TX E-121-R___ GRANT TITLE: Native springsnails and the invasive red-rim melania snail (Melanoides tuberculata), species habitat associations and life history investigations in the San Solomon Spring complex, Texas. REPORTING PERIOD: ____17 Sep 09 to 31 May 12_ OBJECTIVE(S): To determine patterns of abundance, distribution, and habitat use of the Phantom Cave snail (Cochliopa texana), Phantom Spring tryonia (Tryonia cheatumi), and the invasive red-rim melania snail (Melanoides tuberculta) in San Solomon Springs, and potential interactions. Segment Objectives: Task 1. January - February 2010. A reconnaissance visit(s) will be made to the region to investigate the study area and work on specific sampling procedural methods. Visit with TPWD at the Balmorhea State Park, as well as meet The Nature Conservancy personnel at Diamond Y and Sandia springs complexes. Task 2. March 2010– August 2011. Begin sampling. Field sampling will be conducted every 6-8 weeks, over a period of a year and a half. Sampling methods are outlined below stated Tasks. Task 3. December 2010. Completion of first year of study. With four seasonal samples completed, preliminary data analysis and statistical modeling will begin. Preliminary results will be presented at the Texas Chapter of the American Fisheries Society meeting. -

C:\Fish\Eastern Sand Darter Sa.Wpd
EASTERN SAND DARTER STATUS ASSESSMENT Prepared by: David Grandmaison and Joseph Mayasich Natural Resources Research Institute University of Minnesota 5013 Miller Trunk Highway Duluth, MN 55811-1442 and David Etnier Ecology and Evolutionary Biology University of Tennessee 569 Dabney Hall Knoxville, TN 37996-1610 Prepared for: U.S. Fish and Wildlife Service Region 3 1 Federal Drive Fort Snelling, MN 55111 January 2004 NRRI Technical Report No. NRRI/TR-2003/40 DISCLAIMER This document is a compilation of biological data and a description of past, present, and likely future threats to the eastern sand darter, Ammocrypta pellucida (Agassiz). It does not represent a decision by the U.S. Fish and Wildlife Service (Service) on whether this taxon should be designated as a candidate species for listing as threatened or endangered under the Federal Endangered Species Act. That decision will be made by the Service after reviewing this document; other relevant biological and threat data not included herein; and all relevant laws, regulations, and policies. The result of the decision will be posted on the Service's Region 3 Web site (refer to: http://midwest.fws.gov/eco_serv/endangrd/lists/concern.html). If designated as a candidate species, the taxon will subsequently be added to the Service's candidate species list that is periodically published in the Federal Register and posted on the World Wide Web (refer to: http://endangered.fws.gov/wildlife.html). Even if the taxon does not warrant candidate status it should benefit from the conservation recommendations that are contained in this document. ii TABLE OF CONTENTS DISCLAIMER................................................................... -

Darter Reproductive Seasons Author(S): Clark Hubbs Reviewed Work(S): Source: Copeia, Vol
Darter Reproductive Seasons Author(s): Clark Hubbs Reviewed work(s): Source: Copeia, Vol. 1985, No. 1 (Feb. 11, 1985), pp. 56-68 Published by: American Society of Ichthyologists and Herpetologists (ASIH) Stable URL: http://www.jstor.org/stable/1444790 . Accessed: 10/01/2012 14:26 Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at . http://www.jstor.org/page/info/about/policies/terms.jsp JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. American Society of Ichthyologists and Herpetologists (ASIH) is collaborating with JSTOR to digitize, preserve and extend access to Copeia. http://www.jstor.org 56 COPEIA, 1985, NO. 1 changes in kinosternid turtles. J. Herpetol. 6:183- . 1938. Seasonal changes in the testes of the 189. musk turtle Sternotherusodoratus L. J. Morphol. 63: MCPHERSON, R. J., AND K. R. MARION. 1981. Sea- 301-317. sonal testicular cycle of the stinkpot turtle (Ster- SAINTGIRONS, H. 1982. Reproductive cycles of male notherus odoratus) in central Alabama. Herpetolog- snakes and their relationships with climate and fe- ica 37:33-40. male reproductive cycles. Herpetologica 38:5-16. MITCHELL, J. C. 1982. Population ecology and de- SPEAT, R. H. 1973. Seasonal variation in the tubular mography of the freshwater turtles Chrysemyspicta and interstitial areas of the testes in Sternothaerus and Sternotherusodoratus. -
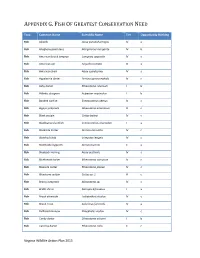
Fish of Greatest Conservation Need
APPENDIX G. FISH OF GREATEST CONSERVATION NEED Taxa Common Name Scientific Name Tier Opportunity Ranking Fish Alewife Alosa pseudoharengus IV a Fish Allegheny pearl dace Margariscus margarita IV b Fish American brook lamprey Lampetra appendix IV c Fish American eel Anguilla rostrata III a Fish American shad Alosa sapidissima IV a Fish Appalachia darter Percina gymnocephala IV c Fish Ashy darter Etheostoma cinereum I b Fish Atlantic sturgeon Acipenser oxyrinchus I b Fish Banded sunfish Enneacanthus obesus IV c Fish Bigeye jumprock Moxostoma ariommum III c Fish Black sculpin Cottus baileyi IV c Fish Blackbanded sunfish Enneacanthus chaetodon I a Fish Blackside darter Percina maculata IV c Fish Blotched chub Erimystax insignis IV c Fish Blotchside logperch Percina burtoni II a Fish Blueback Herring Alosa aestivalis IV a Fish Bluebreast darter Etheostoma camurum IV c Fish Blueside darter Etheostoma jessiae IV c Fish Bluestone sculpin Cottus sp. 1 III c Fish Brassy Jumprock Moxostoma sp. IV c Fish Bridle shiner Notropis bifrenatus I a Fish Brook silverside Labidesthes sicculus IV c Fish Brook Trout Salvelinus fontinalis IV a Fish Bullhead minnow Pimephales vigilax IV c Fish Candy darter Etheostoma osburni I b Fish Carolina darter Etheostoma collis II c Virginia Wildlife Action Plan 2015 APPENDIX G. FISH OF GREATEST CONSERVATION NEED Fish Carolina fantail darter Etheostoma brevispinum IV c Fish Channel darter Percina copelandi III c Fish Clinch dace Chrosomus sp. cf. saylori I a Fish Clinch sculpin Cottus sp. 4 III c Fish Dusky darter Percina sciera IV c Fish Duskytail darter Etheostoma percnurum I a Fish Emerald shiner Notropis atherinoides IV c Fish Fatlips minnow Phenacobius crassilabrum II c Fish Freshwater drum Aplodinotus grunniens III c Fish Golden Darter Etheostoma denoncourti II b Fish Greenfin darter Etheostoma chlorobranchium I b Fish Highback chub Hybopsis hypsinotus IV c Fish Highfin Shiner Notropis altipinnis IV c Fish Holston sculpin Cottus sp. -

Information on the NCWRC's Scientific Council of Fishes Rare
A Summary of the 2010 Reevaluation of Status Listings for Jeopardized Freshwater Fishes in North Carolina Submitted by Bryn H. Tracy North Carolina Division of Water Resources North Carolina Department of Environment and Natural Resources Raleigh, NC On behalf of the NCWRC’s Scientific Council of Fishes November 01, 2014 Bigeye Jumprock, Scartomyzon (Moxostoma) ariommum, State Threatened Photograph by Noel Burkhead and Robert Jenkins, courtesy of the Virginia Division of Game and Inland Fisheries and the Southeastern Fishes Council (http://www.sefishescouncil.org/). Table of Contents Page Introduction......................................................................................................................................... 3 2010 Reevaluation of Status Listings for Jeopardized Freshwater Fishes In North Carolina ........... 4 Summaries from the 2010 Reevaluation of Status Listings for Jeopardized Freshwater Fishes in North Carolina .......................................................................................................................... 12 Recent Activities of NCWRC’s Scientific Council of Fishes .................................................. 13 North Carolina’s Imperiled Fish Fauna, Part I, Ohio Lamprey .............................................. 14 North Carolina’s Imperiled Fish Fauna, Part II, “Atlantic” Highfin Carpsucker ...................... 17 North Carolina’s Imperiled Fish Fauna, Part III, Tennessee Darter ...................................... 20 North Carolina’s Imperiled Fish Fauna, Part -

Endangered Species
FEATURE: ENDANGERED SPECIES Conservation Status of Imperiled North American Freshwater and Diadromous Fishes ABSTRACT: This is the third compilation of imperiled (i.e., endangered, threatened, vulnerable) plus extinct freshwater and diadromous fishes of North America prepared by the American Fisheries Society’s Endangered Species Committee. Since the last revision in 1989, imperilment of inland fishes has increased substantially. This list includes 700 extant taxa representing 133 genera and 36 families, a 92% increase over the 364 listed in 1989. The increase reflects the addition of distinct populations, previously non-imperiled fishes, and recently described or discovered taxa. Approximately 39% of described fish species of the continent are imperiled. There are 230 vulnerable, 190 threatened, and 280 endangered extant taxa, and 61 taxa presumed extinct or extirpated from nature. Of those that were imperiled in 1989, most (89%) are the same or worse in conservation status; only 6% have improved in status, and 5% were delisted for various reasons. Habitat degradation and nonindigenous species are the main threats to at-risk fishes, many of which are restricted to small ranges. Documenting the diversity and status of rare fishes is a critical step in identifying and implementing appropriate actions necessary for their protection and management. Howard L. Jelks, Frank McCormick, Stephen J. Walsh, Joseph S. Nelson, Noel M. Burkhead, Steven P. Platania, Salvador Contreras-Balderas, Brady A. Porter, Edmundo Díaz-Pardo, Claude B. Renaud, Dean A. Hendrickson, Juan Jacobo Schmitter-Soto, John Lyons, Eric B. Taylor, and Nicholas E. Mandrak, Melvin L. Warren, Jr. Jelks, Walsh, and Burkhead are research McCormick is a biologist with the biologists with the U.S. -

ECOLOGY of NORTH AMERICAN FRESHWATER FISHES
ECOLOGY of NORTH AMERICAN FRESHWATER FISHES Tables STEPHEN T. ROSS University of California Press Berkeley Los Angeles London © 2013 by The Regents of the University of California ISBN 978-0-520-24945-5 uucp-ross-book-color.indbcp-ross-book-color.indb 1 44/5/13/5/13 88:34:34 AAMM uucp-ross-book-color.indbcp-ross-book-color.indb 2 44/5/13/5/13 88:34:34 AAMM TABLE 1.1 Families Composing 95% of North American Freshwater Fish Species Ranked by the Number of Native Species Number Cumulative Family of species percent Cyprinidae 297 28 Percidae 186 45 Catostomidae 71 51 Poeciliidae 69 58 Ictaluridae 46 62 Goodeidae 45 66 Atherinopsidae 39 70 Salmonidae 38 74 Cyprinodontidae 35 77 Fundulidae 34 80 Centrarchidae 31 83 Cottidae 30 86 Petromyzontidae 21 88 Cichlidae 16 89 Clupeidae 10 90 Eleotridae 10 91 Acipenseridae 8 92 Osmeridae 6 92 Elassomatidae 6 93 Gobiidae 6 93 Amblyopsidae 6 94 Pimelodidae 6 94 Gasterosteidae 5 95 source: Compiled primarily from Mayden (1992), Nelson et al. (2004), and Miller and Norris (2005). uucp-ross-book-color.indbcp-ross-book-color.indb 3 44/5/13/5/13 88:34:34 AAMM TABLE 3.1 Biogeographic Relationships of Species from a Sample of Fishes from the Ouachita River, Arkansas, at the Confl uence with the Little Missouri River (Ross, pers. observ.) Origin/ Pre- Pleistocene Taxa distribution Source Highland Stoneroller, Campostoma spadiceum 2 Mayden 1987a; Blum et al. 2008; Cashner et al. 2010 Blacktail Shiner, Cyprinella venusta 3 Mayden 1987a Steelcolor Shiner, Cyprinella whipplei 1 Mayden 1987a Redfi n Shiner, Lythrurus umbratilis 4 Mayden 1987a Bigeye Shiner, Notropis boops 1 Wiley and Mayden 1985; Mayden 1987a Bullhead Minnow, Pimephales vigilax 4 Mayden 1987a Mountain Madtom, Noturus eleutherus 2a Mayden 1985, 1987a Creole Darter, Etheostoma collettei 2a Mayden 1985 Orangebelly Darter, Etheostoma radiosum 2a Page 1983; Mayden 1985, 1987a Speckled Darter, Etheostoma stigmaeum 3 Page 1983; Simon 1997 Redspot Darter, Etheostoma artesiae 3 Mayden 1985; Piller et al. -

Aquatic Fish Report
Aquatic Fish Report Acipenser fulvescens Lake St urgeon Class: Actinopterygii Order: Acipenseriformes Family: Acipenseridae Priority Score: 27 out of 100 Population Trend: Unknown Gobal Rank: G3G4 — Vulnerable (uncertain rank) State Rank: S2 — Imperiled in Arkansas Distribution Occurrence Records Ecoregions where the species occurs: Ozark Highlands Boston Mountains Ouachita Mountains Arkansas Valley South Central Plains Mississippi Alluvial Plain Mississippi Valley Loess Plains Acipenser fulvescens Lake Sturgeon 362 Aquatic Fish Report Ecobasins Mississippi River Alluvial Plain - Arkansas River Mississippi River Alluvial Plain - St. Francis River Mississippi River Alluvial Plain - White River Mississippi River Alluvial Plain (Lake Chicot) - Mississippi River Habitats Weight Natural Littoral: - Large Suitable Natural Pool: - Medium - Large Optimal Natural Shoal: - Medium - Large Obligate Problems Faced Threat: Biological alteration Source: Commercial harvest Threat: Biological alteration Source: Exotic species Threat: Biological alteration Source: Incidental take Threat: Habitat destruction Source: Channel alteration Threat: Hydrological alteration Source: Dam Data Gaps/Research Needs Continue to track incidental catches. Conservation Actions Importance Category Restore fish passage in dammed rivers. High Habitat Restoration/Improvement Restrict commercial harvest (Mississippi River High Population Management closed to harvest). Monitoring Strategies Monitor population distribution and abundance in large river faunal surveys in cooperation -
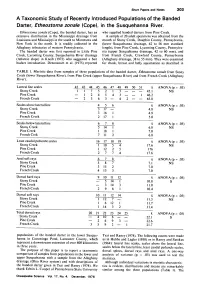
A Taxonomic Study of Recently Introduced Populations of The
Short Papers and Notes 303 A TaxonomicStudy of RecentlyIntroduced Populations of the Banded Darter,Etheostoma zonale (Cope), in the Susquehanna River. Etheostoma zonale (Cope), the banded darter, has an who supplied banded darters from Pine Creek. extensive distribution in the Mississippi drainage from A sample of 20 adult specimens was obtained from the Louisiana and Mississippi in the south to Minnesota and mouth of Stony Creek, Dauphin County, Pennsylvania, New York in the north. It is readily collected in the (lower Susquehanna drainage, 42 to 56 mm standard Allegheny tributaries of western Pennsylvania. length), from Pine Creek, Lycoming County, Pennsylva- The banded darter was first reported in Little Pine nia (upper Susquehanna drainage, 42 to 60 mm), and Creek, Lycoming County, Susquehanna River drainage from French Creek, Crawford County, Pennsylvania (Atlantic slope) in Kneib (1972) who suggested a bait (Allegheny drainage, 38 to 55 mm). They were examined bucket introduction. Denoncourt et al. (1975) reported for cheek, breast and belly squamation as described in TABLE 1. Meristic data from samples of three populations of the banded darter, Etheostoma zonale from Stony Creek (lower Susquehanna River), from Pine Creek (upper Susquehanna River) and from French Creek (Allegheny River). Lateral line scales 42 43 44 45 46 47 48 49 50 51 k ANOVA (p = .05) Stony Creek 1 1 7 5 2 1 3 - -- 45.1 NS Pine Creek - 1 2 4 5 4 3 - - 1 46.2 French Creek - 2 3 6 3 - 4 2 - - 45.8 Scales above lateral line 4 5 6 R ANOVA (p = .05) Stony Creek 3 -

Summary Report of Freshwater Nonindigenous Aquatic Species in U.S
Summary Report of Freshwater Nonindigenous Aquatic Species in U.S. Fish and Wildlife Service Region 4—An Update April 2013 Prepared by: Pam L. Fuller, Amy J. Benson, and Matthew J. Cannister U.S. Geological Survey Southeast Ecological Science Center Gainesville, Florida Prepared for: U.S. Fish and Wildlife Service Southeast Region Atlanta, Georgia Cover Photos: Silver Carp, Hypophthalmichthys molitrix – Auburn University Giant Applesnail, Pomacea maculata – David Knott Straightedge Crayfish, Procambarus hayi – U.S. Forest Service i Table of Contents Table of Contents ...................................................................................................................................... ii List of Figures ............................................................................................................................................ v List of Tables ............................................................................................................................................ vi INTRODUCTION ............................................................................................................................................. 1 Overview of Region 4 Introductions Since 2000 ....................................................................................... 1 Format of Species Accounts ...................................................................................................................... 2 Explanation of Maps ................................................................................................................................ -

Comal County Regional Habitat Conservation Plan Environmental Impact Statement
Draft Comal County Regional Habitat Conservation Plan Environmental Impact Statement Prepared for: Comal County, Texas Comal County Commissioners Court Prepared by: SWCA Environmental Consultants Smith, Robertson, Elliott, Glen, Klein & Bell, L.L.P. Prime Strategies, Inc. Texas Perspectives, Inc. Capital Market Research, Inc. April 2010 SWCA Project Number 12659-139-AUS DRAFT COMAL COUNTY REGIONAL HABITAT CONSERVATION PLAN ENVIRONMENTAL IMPACT STATEMENT April 2010 Type of Action: Administrative Lead Agency: U.S. Department of the Interior Fish and Wildlife Service Responsible Official: Adam Zerrenner Field Supervisor U.S. Fish and Wildlife Service 10711 Burnet Road, Suite 200 Austin, Texas For Information: Bill Seawell Fish and Wildlife Biologist U.S. Fish and Wildlife Service 10711 Burnet Road, Suite 200 Austin, Texas Tele: 512-490-0057 Abstract: Comal County, Texas, is applying for an incidental take permit (Permit) under section 10(a)(1)(B) of the Endangered Species Act of 1973, as amended 16 U.S.C. § 1531, et seq. (ESA), to authorize the incidental take of two endangered species, the golden-cheeked warbler (Dendroica chrysoparia) and the black-capped vireo (Vireo atricapilla), referred to collectively as the “Covered Species.” In support of the Permit application, the County has prepared a regional habitat conservation plan (Proposed RHCP), covering a 30-year period from 2010 to 2040. The Permit Area for the Proposed RHCP and the area of potential effect for this Environmental Impact Statement (EIS) is Comal County in central Texas. The requested Permit would authorize the following incidental take and mitigation for the golden-cheeked warbler: Take: As conservation credits are created through habitat preservation, authorize up to 5,238 acres (2,120 hectares) of golden-cheeked warbler habitat to be impacted over the 30-year life of the Proposed RHCP.