Identification of a Vesicular Nucleotide Transporter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Regulatory Effects of Cu, Zn, and Ca on Fe Absorption: the Intricate Play Between Nutrient Transporters
Nutrients 2013, 5, 957-970; doi:10.3390/nu5030957 OPEN ACCESS nutrients ISSN 2072-6643 www.mdpi.com/journal/nutrients Review Regulatory Effects of Cu, Zn, and Ca on Fe Absorption: The Intricate Play between Nutrient Transporters Nathalie Scheers Division of Life Sciences, Food Science, Department of Chemical and Biological Engineering, Chalmers University of Technology, SE-412 96 Gothenburg, Sweden; E-Mail: [email protected]; Tel.: +46-31-772-3821; Fax: +46-31-772-3830 Received: 4 February 2013; in revised form: 8 March 2013 / Accepted: 15 March 2013 / Published: 20 March 2013 Abstract: Iron is an essential nutrient for almost every living organism because it is required in a number of biological processes that serve to maintain life. In humans, recycling of senescent erythrocytes provides most of the daily requirement of iron. In addition, we need to absorb another 1–2 mg Fe from the diet each day to compensate for losses due to epithelial sloughing, perspiration, and bleeding. Iron absorption in the intestine is mainly regulated on the enterocyte level by effectors in the diet and systemic regulators accessing the enterocyte through the basal lamina. Recently, a complex meshwork of interactions between several trace metals and regulatory proteins was revealed. This review focuses on advances in our understanding of Cu, Zn, and Ca in the regulation of iron absorption. Ascorbate as an important player is also considered. Keywords: iron; absorption; Fe; Zn; Cu; Ca; ascorbate 1. Introduction Iron (Fe) is the second most abundant metal on earth and is a necessity for all life. Iron plays the key role in numerous enzymatic reactions due to its ease in shifting between the two common oxidation states, ferrous (Fe2+) and ferric (Fe3+) iron. -

Identification of a Novel Nucleobase-Ascorbate Transporter
bioRxiv preprint doi: https://doi.org/10.1101/287870; this version posted December 7, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 Identification of a novel Nucleobase-Ascorbate 2 Transporter family member in fish and amphibians 3 Diogo Oliveira*,1, André M. Machado*,1, Tiago Cardoso1, Mónica Lopes-Marques1, L. Filipe 4 C. Castro1,2● and Raquel Ruivo1● 5 1CIIMAR – Interdisciplinary Centre of Marine and Environmental Research, U. Porto – University 6 of Porto, Porto, Portugal 7 2Department of Biology, Faculty of Sciences, U. Porto - University of Porto, Portugal 8 *Equal contribution 9 ●Corresponding authors at: CIIMAR, Terminal de Cruzeiros do Porto de Leixões, Av. General 10 Norton de Matos s/n, 4450-208 Matosinhos, Portugal. Tel.: +351 223 401 831 11 12 Running title: Novel uric acid transporter in fish and amphibians 13 14 15 bioRxiv preprint doi: https://doi.org/10.1101/287870; this version posted December 7, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 16 Abstract:Nucleobase-Ascorbate Transporter (NAT) family includes ascorbic acid, nucleobases 17 and uric acid transporters: with a broad evolutionary distribution. In vertebrates, four members 18 have been previously recognized, the ascorbate transporters Slc23a1 and Slc3a2, the nucleobase 19 transporter Slc23a4 and an orphan transporter Slc23a3. -
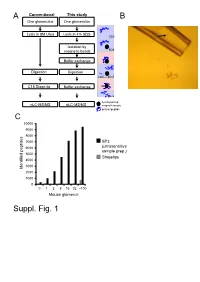
Suppl. Fig. 1 A
A Conventional This study B One glomerulus One glomerulus Lysis in 8M Urea Lysis in 4% SDS SDS Isolation by magnetic beads SDS Buffer exchange Digestion Digestion Try LysC pH>7 C18 Stage tip Buffer exchange pH<4 functionalized nLC-MS/MS nLC-MS/MS magnetic beads protein/peptide C 10000 9000 8000 7000 SP3 6000 (ultrasensitive sample prep.) 5000 Stagetips 4000 3000 Identified peptides 2000 1000 0 0 1 2 8 16 32 ~100 Mouse glomeruli Suppl. Fig. 1 S1. Sample preparation methods. A. Scheme of conventional (C18 Stage Tip) and ultrasensitive proteomic sample preparation methods. B. Manual microdissection and isolation of single glomeruli. The arrow indicates a single isolated glomerulus subjected to proteome analysis. The tip capacity is 10µl. C. Stagetips, a conventional proteomic sample preparation method, is compared with ultrasensitive (SP3) proteome analysis. The indicated amounts of mouse glomeruli were prepared by the respective sample preparation protocols, and peptide numbers after nLC-MS/MS are plotted (all FDR<0.01). A 100 1 Tubule B 80 60 40 Intensity CCD 20 40 0 100 20 S1 TAL 80 0 Tubule proximal tubule Loading... 60 0 Intensity 40 S2 20 -20 control Component 2 (22.5%) S3 0 0 10 20 30 40 50 60 -40 -20 0 20 40 Time (min) Component 2 (47.1%) C D 6000 Mouse proximal tubule 5000 8 4000 3000 7 (iBAQ) Loading... 10 2000 log 6 Number of peptides 1000 0 5 Control mouse mouse mouse human proximal mTAL CCD proximal 0 1500 tubule tubule Rank protein (S1) (S1) R=0.7 E F 8 ACTB Human proximal tubule 8 7 SLC9A3R1 ATP1A1 SLC3A2 SLC25A5 ATP1B1 7 SLC5A2 SLC25A3 CUBN CLTC SLC27A2 SLC5A12 LRP2 6 SLC25A13 SLC25A10 SLC5A1 SLC22A6 6 SLC25A4 Loading.. -

The Concise Guide to Pharmacology 2019/20
Edinburgh Research Explorer THE CONCISE GUIDE TO PHARMACOLOGY 2019/20 Citation for published version: Cgtp Collaborators 2019, 'THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Transporters', British Journal of Pharmacology, vol. 176 Suppl 1, pp. S397-S493. https://doi.org/10.1111/bph.14753 Digital Object Identifier (DOI): 10.1111/bph.14753 Link: Link to publication record in Edinburgh Research Explorer Document Version: Publisher's PDF, also known as Version of record Published In: British Journal of Pharmacology General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 28. Sep. 2021 S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2019/20: Transporters. British Journal of Pharmacology (2019) 176, S397–S493 THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Transporters Stephen PH Alexander1 , Eamonn Kelly2, Alistair Mathie3 ,JohnAPeters4 , Emma L Veale3 , Jane F Armstrong5 , Elena Faccenda5 ,SimonDHarding5 ,AdamJPawson5 , Joanna L -

Towards the Elucidation of Orphan Lysosomal Transporters Quentin Verdon
Towards the Elucidation of Orphan Lysosomal Transporters Quentin Verdon To cite this version: Quentin Verdon. Towards the Elucidation of Orphan Lysosomal Transporters. Cancer. Université Paris Saclay (COmUE), 2016. English. NNT : 2016SACLS144. tel-01827233 HAL Id: tel-01827233 https://tel.archives-ouvertes.fr/tel-01827233 Submitted on 2 Jul 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. NNT : 2016SACLS144 THESE DE DOCTORAT DE L’UNIVERSITE PARIS-SACLAY PREPAREE A L’UNIVERSITE PARIS-SUD ECOLE DOCTORALE N°568 BIOSIGNE | Signalisations et réseaux intégratifs en biologie Spécialité de doctorat : aspects moléculaires et cellulaires de la biologie Par Mr Quentin Verdon Towards the elucidation of orphan lysosomal transporters: several shots on target and one goal Thèse présentée et soutenue à Paris le 29/06/2016 » : Composition du Jury : Mr Le Maire Marc Professeur, Université Paris-Sud Président Mr Birman Serge Directeur de recherche, CNRS Rapporteur Mr Murray James Assistant professor, Trinity college Dublin Rapporteur Mr Goud Bruno Directeur de recherche, CNRS Examinateur Mr Gasnier Bruno Directeur de recherche, CNRS Directeur de thèse Mme Sagné Corinne Chargée de recherche, INSERM Co-directeur de thèse Table of contents Remerciements (acknowledgements) 6 Abbreviations 7 Abstracts 10 Introduction 12 1 Physiology of lysosomes 12 1.1 Discovery and generalities 12 1.2 Degradative function 13 1.3. -

Toxicological Profile for Nitrate and Nitrite
NITRATE AND NITRITE 29 3. HEALTH EFFECTS 3.1 INTRODUCTION The primary purpose of this chapter is to provide public health officials, physicians, toxicologists, and other interested individuals and groups with an overall perspective on the toxicology of nitrate and nitrite. It contains descriptions and evaluations of toxicological studies and epidemiological investigations and provides conclusions, where possible, on the relevance of toxicity and toxicokinetic data to public health. A glossary and list of acronyms, abbreviations, and symbols can be found at the end of this profile. 3.2 DISCUSSION OF HEALTH EFFECTS BY ROUTE OF EXPOSURE To help public health professionals and others address the needs of persons living or working near hazardous waste sites, the information in this section is organized first by route of exposure (inhalation, oral, and dermal) and then by health effect (e.g., death, systemic, immunological, neurological, reproductive, developmental, and carcinogenic effects). These data are discussed in terms of three exposure periods: acute (14 days or less), intermediate (15–364 days), and chronic (365 days or more). Levels of significant exposure for each route and duration are presented in tables and illustrated in figures. The points in the figures showing no-observed-adverse-effect levels (NOAELs) or lowest- observed-adverse-effect levels (LOAELs) reflect the actual doses (levels of exposure) used in the studies. LOAELs have been classified into "less serious" or "serious" effects. "Serious" effects are those that evoke failure in a biological system and can lead to morbidity or mortality (e.g., acute respiratory distress or death). "Less serious" effects are those that are not expected to cause significant dysfunction or death, or those whose significance to the organism is not entirely clear. -

Sialic Acid Storage Disease
Sialic acid storage disease Description Sialic acid storage disease is an inherited disorder that primarily affects the nervous system. People with sialic acid storage disease have signs and symptoms that may vary widely in severity. This disorder is generally classified into one of three forms: infantile free sialic acid storage disease, Salla disease, and intermediate severe Salla disease. Infantile free sialic acid storage disease (ISSD) is the most severe form of this disorder. Babies with this condition have severe developmental delay, weak muscle tone ( hypotonia), and failure to gain weight and grow at the expected rate (failure to thrive). They may have unusual facial features that are often described as "coarse," seizures, bone malformations, an enlarged liver and spleen (hepatosplenomegaly), and an enlarged heart (cardiomegaly). The abdomen may be swollen due to the enlarged organs and an abnormal buildup of fluid in the abdominal cavity (ascites). Affected infants may have a condition called hydrops fetalis in which excess fluid accumulates in the body before birth. Children with this severe form of the condition usually live only into early childhood. Salla disease is a less severe form of sialic acid storage disease. Babies with Salla disease usually begin exhibiting hypotonia during the first year of life and go on to experience progressive neurological problems. Signs and symptoms of Salla disease include intellectual disability and developmental delay, seizures, problems with movement and balance (ataxia), abnormal tensing of the muscles (spasticity), and involuntary slow, sinuous movements of the limbs (athetosis). Individuals with Salla disease usually survive into adulthood. People with intermediate severe Salla disease have signs and symptoms that fall between those of ISSD and Salla disease in severity. -

Sialin (SLC17A5) Functions As a Nitrate Transporter in the Plasma Membrane
Sialin (SLC17A5) functions as a nitrate transporter in the plasma membrane Lizheng Qina,1, Xibao Liub,1, Qifei Suna, Zhipeng Fana, Dengsheng Xiaa, Gang Dinga, Hwei Ling Ongb, David Adamsc, William A. Gahlc, Changyu Zhengb, Senrong Qia, Luyuan Jina, Chunmei Zhanga, Liankun Gud, Junqi Hee, Dajun Dengd,2, Indu S. Ambudkarb,2, and Songlin Wanga,e,2 aSalivary Gland Disease Center and Beijing Key Laboratory of Tooth Regeneration and Function Reconstruction, Capital Medical University School of Stomatology, Beijing 100050, China; bMolecular Physiology and Therapeutics Branch, National Institute of Dental and Craniofacial Research, Bethesda, MD 20892; cMedical Genetics Branch, National Human Genome Research Institute, Bethesda, MD 20892; dKey Laboratory of Carcinogenesis and Translational Research, Peking University Cancer Hospital and Institute, Beijing 100142, China; and eDepartment of Biochemistry and Molecular Biology, Capital Medical University School of Basic Medicine, Beijing 100069, China Edited by Mark Gladwin, University of Pittsburg Medical Center, Pittsburgh, PA and accepted by the Editorial Board June 5, 2012 (received for review October 13, 2011) In vivo recycling of nitrate (NO −) and nitrite (NO −) is an important Nitrate uptake into salivary glands represents the key initial step 3 2 − alternative pathway for the generation of nitric oxide (NO) and in NO clearance from the serum; however, the mechanism me- 3 − maintenance of systemic nitrate–nitrite–NO balance. More than diating transport of NO3 in salivary gland epithelial cells has not − − fl 25% of the circulating NO3 is actively removed and secreted by yet been established. In this study, we examined NO3 in ux in − + salivary glands. Oral commensal bacteria convert salivary NO3 to salivary gland cells. -

Potential Roles of Nitrate and Nitrite in Nitric Oxide Metabolism in the Eye Ji Won Park1, Barbora Piknova1, Audrey Jenkins2, David Hellinga2, Leonard M
www.nature.com/scientificreports OPEN Potential roles of nitrate and nitrite in nitric oxide metabolism in the eye Ji Won Park1, Barbora Piknova1, Audrey Jenkins2, David Hellinga2, Leonard M. Parver3 & Alan N. Schechter1* Nitric oxide (NO) signaling has been studied in the eye, including in the pathophysiology of some eye diseases. While NO production by nitric oxide synthase (NOS) enzymes in the eye has been − characterized, the more recently described pathways of NO generation by nitrate ( NO3 ) and nitrite − (NO2 ) ions reduction has received much less attention. To elucidate the potential roles of these pathways, we analyzed nitrate and nitrite levels in components of the eye and lacrimal glands, primarily in porcine samples. Nitrate and nitrite levels were higher in cornea than in other eye parts, while lens contained the least amounts. Lacrimal glands exhibited much higher levels of both ions compared to other organs, such as liver and skeletal muscle, and even to salivary glands which are known to concentrate these ions. Western blotting showed expression of sialin, a known nitrate transporter, in the lacrimal glands and other eye components, and also xanthine oxidoreductase, a nitrate and nitrite reductase, in cornea and sclera. Cornea and sclera homogenates possessed a measurable amount of nitrate reduction activity. These results suggest that nitrate ions are concentrated in the lacrimal glands by sialin and can be secreted into eye components via tears and then reduced to nitrite and NO, thereby being an important source of NO in the eye. Te NO generation pathway from L-arginine by endogenous NOS enzymes under normoxic conditions has been central in identifying the physiological roles of NO in numerous biological processes1. -

Glutamate, Aspartate and Nucleotide Transporters in the SLC17 Family Form Four Main Phylogenetic Clusters: Evolution and Tissue
Sreedharan et al. BMC Genomics 2010, 11:17 http://www.biomedcentral.com/1471-2164/11/17 RESEARCH ARTICLE Open Access Glutamate, aspartate and nucleotide transporters in the SLC17 family form four main phylogenetic clusters: evolution and tissue expression Smitha Sreedharan1, Jafar HA Shaik1, Pawel K Olszewski1,2, Allen S Levine2,3, Helgi B Schiöth1, Robert Fredriksson1* Abstract Background: The SLC17 family of transporters transports the amino acids: glutamate and aspartate, and, as shown recently, also nucleotides. Vesicular glutamate transporters are found in distinct species, such as C. elegans, but the evolutionary origin of most of the genes in this family has been obscure. Results: Our phylogenetic analysis shows that the SLC17 family consists of four main phylogenetic clades which were all present before the divergence of the insect lineage. One of these clades has not been previously described and it is not found in vertebrates. The clade containing Slc17a9 had the most restricted evolutionary history with only one member in most species. We detected expression of Slc17a1-17a4 only in the peripheral tissues but not in the CNS, while Slc17a5- Slc17a9 are highly expressed in both the CNS and periphery. Conclusions: The in situ hybridization studies on vesicular nucleotide transporter revealed high expression throughout the cerebral cortex, certain areas in the hippocampus and in specific nuclei of the hypothalamus and thalamus. Some of the regions with high expression, such as the medial habenula and the dentate gyrus of the hippocampus, are important sites for purinergic neurotransmission. Noteworthy, other areas relying on purine- mediated signaling, such as the molecular layer of the dentate gyrus and the periaqueductal gray, lack or have a very low expression of Slc17a9, suggesting that there could be another nucleotide transporter in these regions. -

Characterization of Centrally Expressed Solute Carriers
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Medicine 1215 Characterization of Centrally Expressed Solute Carriers Histological and Functional Studies with Transgenic Mice SAHAR ROSHANBIN ACTA UNIVERSITATIS UPSALIENSIS ISSN 1651-6206 ISBN 978-91-554-9555-8 UPPSALA urn:nbn:se:uu:diva-282956 2016 Dissertation presented at Uppsala University to be publicly examined in B:21, Husargatan. 75124 Uppsala, Uppsala, Friday, 3 June 2016 at 13:15 for the degree of Doctor of Philosophy (Faculty of Medicine). The examination will be conducted in English. Faculty examiner: Biträdande professor David Engblom (Institutionen för klinisk och experimentell medicin, Cellbiologi, Linköpings Universitet). Abstract Roshanbin, S. 2016. Characterization of Centrally Expressed Solute Carriers. Histological and Functional Studies with Transgenic Mice. (. His). Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Medicine 1215. 62 pp. Uppsala: Acta Universitatis Upsaliensis. ISBN 978-91-554-9555-8. The Solute Carrier (SLC) superfamily is the largest group of membrane-bound transporters, currently with 456 transporters in 52 families. Much remains unknown about the tissue distribution and function of many of these transporters. The aim of this thesis was to characterize select SLCs with emphasis on tissue distribution, cellular localization, and function. In paper I, we studied the leucine transporter B0AT2 (Slc6a15). Localization of B0AT2 and Slc6a15 in mouse brain was determined using in situ hybridization (ISH) and immunohistochemistry (IHC), localizing it to neurons, epithelial cells, and astrocytes. Furthermore, we observed a lower reduction of food intake in Slc6a15 knockout mice (KO) upon intraperitoneal injections with leucine, suggesting B0AT2 is involved in mediating the anorexigenic effects of leucine. -

Classic Ketogenic Diet and Modified Atkins Diet in SLC2A1 Positive And
nutrients Article Classic Ketogenic Diet and Modified Atkins Diet in SLC2A1 Positive and Negative Patients with Suspected GLUT1 Deficiency Syndrome: A Single Center Analysis of 18 Cases Jana Ruiz Herrero 1,* , Elvira Cañedo Villarroya 2 , Luis González Gutiérrez-Solana 3,4 , Beatriz García Alcolea 2, Begoña Gómez Fernández 2, Laura Andrea Puerta Macfarland 2 and Consuelo Pedrón-Giner 2 1 Department of Pediatric Gastroenterology, Pediatric Service, San Rafael Hospital, 28016 Madrid, Spain 2 Department of Gastroenterology and Nutrition, University Children’s Hospital Niño Jesús, 28009 Madrid, Spain; [email protected] (E.C.V.); [email protected] (B.G.A.); [email protected] (B.G.F.); [email protected] (L.A.P.M.); [email protected] (C.P.-G.) 3 Department of Neurology, University Children’s Hospital Niño Jesús, 28009 Madrid, Spain; [email protected] 4 Center for Biomedical Network Research on Rare Diseases (CIBERER), Health Institute Carlos III, 28029 Madrid, Spain * Correspondence: [email protected]; Tel.: +34-915-035-933 Citation: Ruiz Herrero, J.; Cañedo Villarroya, E.; González Abstract: Background: Glucose transporter type 1 deficiency syndrome (GLUT1DS) is caused Gutiérrez-Solana, L.; García Alcolea, by mutations in the SLC2A1 gene and produces seizures, neurodevelopmental impairment, and B.; Gómez Fernández, B.; Puerta Macfarland, L.A.; Pedrón-Giner, C. movement disorders. Ketogenic dietary therapies (KDT) are the gold standard treatment. Similar Classic Ketogenic Diet and Modified symptoms may appear in SLC2A1 negative patients. The purpose is to evaluate the effectiveness Atkins Diet in SLC2A1 Positive and of KDT in children with GLUT1DS suspected SLC2A1 (+) and (-), side effects (SE), and the impact Negative Patients with Suspected on patients nutritional status.