Engineering Thermodynamics By
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

3-1 Adiabatic Compression
Solution Physics 213 Problem 1 Week 3 Adiabatic Compression a) Last week, we considered the problem of isothermal compression: 1.5 moles of an ideal diatomic gas at temperature 35oC were compressed isothermally from a volume of 0.015 m3 to a volume of 0.0015 m3. The pV-diagram for the isothermal process is shown below. Now we consider an adiabatic process, with the same starting conditions and the same final volume. Is the final temperature higher, lower, or the same as the in isothermal case? Sketch the adiabatic processes on the p-V diagram below and compute the final temperature. (Ignore vibrations of the molecules.) α α For an adiabatic process ViTi = VfTf , where α = 5/2 for the diatomic gas. In this case Ti = 273 o 1/α 2/5 K + 35 C = 308 K. We find Tf = Ti (Vi/Vf) = (308 K) (10) = 774 K. b) According to your diagram, is the final pressure greater, lesser, or the same as in the isothermal case? Explain why (i.e., what is the energy flow in each case?). Calculate the final pressure. We argued above that the final temperature is greater for the adiabatic process. Recall that p = nRT/ V for an ideal gas. We are given that the final volume is the same for the two processes. Since the final temperature is greater for the adiabatic process, the final pressure is also greater. We can do the problem numerically if we assume an idea gas with constant α. γ In an adiabatic processes, pV = constant, where γ = (α + 1) / α. -

Thermodynamics Formulation of Economics Burin Gumjudpai A
Thermodynamics Formulation of Economics 306706052 NIDA E-THESIS 5710313001 thesis / recv: 09012563 15:07:41 seq: 16 Burin Gumjudpai A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Economics (Financial Economics) School of Development Economics National Institute of Development Administration 2019 Thermodynamics Formulation of Economics Burin Gumjudpai School of Development Economics Major Advisor (Associate Professor Yuthana Sethapramote, Ph.D.) The Examining Committee Approved This Thesis Submitted in Partial 306706052 Fulfillment of the Requirements for the Degree of Master of Economics (Financial Economics). Committee Chairperson NIDA E-THESIS 5710313001 thesis / recv: 09012563 15:07:41 seq: 16 (Assistant Professor Pongsak Luangaram, Ph.D.) Committee (Assistant Professor Athakrit Thepmongkol, Ph.D.) Committee (Associate Professor Yuthana Sethapramote, Ph.D.) Dean (Associate Professor Amornrat Apinunmahakul, Ph.D.) ______/______/______ ABST RACT ABSTRACT Title of Thesis Thermodynamics Formulation of Economics Author Burin Gumjudpai Degree Master of Economics (Financial Economics) Year 2019 306706052 We consider a group of information-symmetric consumers with one type of commodity in an efficient market. The commodity is fixed asset and is non-disposable. We are interested in seeing if there is some connection of thermodynamics formulation NIDA E-THESIS 5710313001 thesis / recv: 09012563 15:07:41 seq: 16 to microeconomics. We follow Carathéodory approach which requires empirical existence of equation of state (EoS) and coordinates before performing maximization of variables. In investigating EoS of various system for constructing the economics EoS, unexpectedly new insights of thermodynamics are discovered. With definition of truly endogenous function, criteria rules and diagrams are proposed in identifying the status of EoS for an empirical equation. -
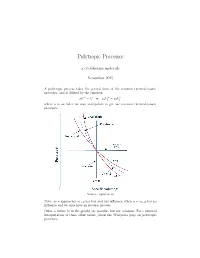
Polytropic Processes
Polytropic Processes a.cyclohexane.molecule November 2015 A polytropic process takes the general form of the common thermodynamic processes, and is defined by the equation n n n pV = C or p1V1 = p2V2 where n is an index we may manipulate to get our common thermodynamic processes. Source: nptel.ac.in Note: as n approaches 1, p has less and less influence; when n = 1, p has no influence and we thus have an isobaric process. Other n values (x in the graph) are possible, but not common. For a physical interpretation of these other values, check the Wikipedia page on polytropic processes. Because polytropic processes are general representations of thermodynamic pro- cesses, any quantities we calculate for a polytropic process will be a general equation for that quantity in all other thermodynamic processes. We proceed to find the work and heat associated with a polytropic process. We start with the definition of work: Z V2 w = − p dV V1 The polytropic equation pV n = C allows us to manipulate our formula for work in the following manner, as long as n 6= 1 V V Z 2 C Z 2 C V C V w = − dV = −C V −n dV = − V 1−n 2 = V 1−n 2 n V1 V1 V1 V V1 1 − n n − 1 Evaluating this expression, C CV 1−n − CV 1−n w = V 1−n − V 1−n = 2 1 n − 1 2 1 n − 1 n n We let the first C be p2V2 and the second C be p1V1 , such that the exponents cancel to obtain p V nV 1−n − p V nV 1−n p V − p V R(T − T ) w = 2 2 2 1 1 1 = 2 2 1 1 = 2 1 n − 1 n − 1 n − 1 where the last equality follows from the ideal gas law pV = RT and our deriva- tion for the work of a polytropic process is complete. -

MECH 230 – Thermodynamics 1 Final Workbook Solutions
MECH 230 – Thermodynamics 1 Final Workbook Solutions CREATED BY JUSTIN BONAL MECH 230 Final Exam Workbook Contents 1.0 General Knowledge ........................................................................................................................... 1 1.1 Unit Analysis .................................................................................................................................. 1 1.2 Pressure ......................................................................................................................................... 1 2.0 Energies ............................................................................................................................................ 2 2.1 Work .............................................................................................................................................. 2 2.2 Internal Energy .............................................................................................................................. 3 2.3 Heat ............................................................................................................................................... 3 2.4 First Law of Thermodynamics ....................................................................................................... 3 3.0 Ideal gas ............................................................................................................................................ 4 3.1 Universal Gas Constant ................................................................................................................. -

Speech Sound Programme for 'K' / 'C' at the Start of Words
Speech Sound Programme for ‘k’ / ‘c’ at the start of words This programme is for children who are producing a ‘t’ sound in place of a ‘k’ / ‘c’ sound at the start of words. For example, ‘cat’ is produced ‘tat’ and ‘cup’ is produced ‘tup’. ‘k’ is the target sound. ‘t’ is the produced sound. Below are a set of stages to work through with the child. Start with Stage 1 and only move up to the next stage when you are confident that the child has achieved the current stage. It is recommended that the programme is carried out for 15 minutes three times a week. If you would like any advice or you feel that the child is not making progress, please contact the Speech and Language Therapy team on 0151 514 2334. Stage 1 - Listening for the target sound 1. See ‘Picture Set 1’ for sound cue pictures that represent the target sound ‘k’ and the produced sound ‘t’. 2. Place the two pictures (‘k’ and ‘t’) in front of the child. 3. Teach the child the sounds, not the letter names, e.g., say ‘k’ and not ‘k-uh’. 4. Say the two sounds at random and ask the child to point or place a counter on which sound they hear. 5. Repeat this activity a number of times until you are sure the child can consistently hear the difference between the two sounds. Stage 2 - Sorting pictures by their first sound. 1. Now that the child can hear the difference between the two sounds (‘k’ and ‘t’) on their own, try the same with words. -

Fuel Cells Versus Heat Engines: a Perspective of Thermodynamic and Production
Fuel Cells Versus Heat Engines: A Perspective of Thermodynamic and Production Efficiencies Introduction: Fuel Cells are being developed as a powering method which may be able to provide clean and efficient energy conversion from chemicals to work. An analysis of their real efficiencies and productivity vis. a vis. combustion engines is made in this report. The most common mode of transportation currently used is gasoline or diesel engine powered automobiles. These engines are broadly described as internal combustion engines, in that they develop mechanical work by the burning of fossil fuel derivatives and harnessing the resultant energy by allowing the hot combustion product gases to expand against a cylinder. This arrangement allows for the fuel heat release and the expansion work to be performed in the same location. This is in contrast to external combustion engines, in which the fuel heat release is performed separately from the gas expansion that allows for mechanical work generation (an example of such an engine is steam power, where fuel is used to heat a boiler, and the steam then drives a piston). The internal combustion engine has proven to be an affordable and effective means of generating mechanical work from a fuel. However, because the majority of these engines are powered by a hydrocarbon fossil fuel, there has been recent concern both about the continued availability of fossil fuels and the environmental effects caused by the combustion of these fuels. There has been much recent publicity regarding an alternate means of generating work; the hydrogen fuel cell. These fuel cells produce electric potential work through the electrochemical reaction of hydrogen and oxygen, with the reaction product being water. -

3. Thermodynamics Thermodynamics
Design for Manufacture 3. Thermodynamics Thermodynamics • Thermodynamics – study of heat related matter in motion. • Ma jor deve lopments d uri ng 1650 -1850 • Engineering thermodynamics mainly concerned with work produc ing or utili si ng machi nes such as - Engines, - TbiTurbines and - Compressors together w ith th e worki ng sub stances used i n th e machi nes. • Working substance – fluids: capable of deformation, energy transfer • Air an d s team are common wor king sub st ance Design and Manufacture Pressure F P = A Force per unit area. Unit: Pascal (Pa) N / m 2 1 Bar =105 Pa 1 standard atmosphere = 1.01325 Bar 1 Bar =14.504 Psi (pound force / square inch, 1N= 0.2248 pound force) 1 Psi= 6894.76 Pa Design and Manufacture Phase Nature of substance. Matter can exists in three phases: solid, liquid and gas Cycle If a substance undergoes a series of processes and return to its original state, then it is said to have been taken through a cycle. Design for Manufacture Process A substance is undergone a process if the state is changed by operation carried out on it Isothermal process - Constant temperature process Isobaric process - Constant pressure process Isometric process or isochoric process - Constant volume process Adiabatic Process No heat is transferred, if a process happens so quickly that there is no time to transfer heat, or the system is very well insulated from its surroundings. Polytropic process Occurs with an interchange of both heat and work between the system and its surroundings E.g. Nonadiabatic expansion or compression -

Changes in the Risk of Stroke in Dialysis Patients: a Retrospective Analysis Over the Last 40 Years
toxins Article Changes in the Risk of Stroke in Dialysis Patients: A Retrospective Analysis over the Last 40 Years Toshiya Aono 1,†, Yuki Shinya 1,2,*,† , Satoru Miyawaki 2 , Takehiro Sugiyama 3,4, Isao Kumagai 5, Atsumi Takenobu 1, Masahiro Shin 2 , Nobuhito Saito 2 and Akira Teraoka 1 1 Department of Neurosurgery, Teraoka Memorial Hospital, Hiroshima 729-3103, Japan; [email protected] (T.A.); [email protected] (A.T.); [email protected] (A.T.) 2 Department of Neurosurgery, The University of Tokyo Hospital, Tokyo 113-8655, Japan; [email protected] (S.M.); [email protected] (M.S.); [email protected] (N.S.) 3 Diabetes and Metabolism Information Center, Research Institute, National Center for Global Health and Medicine, Tokyo 162-8655, Japan; [email protected] 4 Department of Health Services Research, Faculty of Medicine, University of Tsukuba, Ibaraki 305-8575, Japan 5 Department of Nephrology, Teraoka Memorial Hospital, Hiroshima 729-3103, Japan; [email protected] * Correspondence: [email protected]; Tel.: +81-3-5800-8853 † These two authors have contributed equally to this study and are thus co-first authors. Abstract: The stroke incidence in hemodialysis (HD) patients is high, but the associated factors remain largely unknown. This study aimed to analyze stroke incidence in HD patients and changes in risk factors. Data of 291 patients were retrospectively analyzed. The cumulative stroke incidences were 21.6% at 10 years and 31.5% at 20. Diabetic nephropathy (DN) significantly increased overall stroke (hazard ratio (HR), 2.24; 95% confidence interval (CI), 1.21–4.12; p = 0.001) and ischemic p stroke (HR, 2.16; 95% CI, 1.00–4.64; = 0.049). -

[email protected]
Fundamentals of mechanical engineering [email protected] MOHAMMED ABASS ALI What is thermodynamics Thermodynamics is the branch of physics that studies the effects of temperature and heat on physical systems at the macroscopic scale. In addition, it also studies the relationship that exists between heat, work and energy. Thermal energy is found in many forms in today’s society including power generation of electricity using gas, coal or nuclear, heating water by gas or electric. THE BASICS OF THERMODYNAMICS Basic concepts Properties are Features of a system which include mass, volume, energy, pressure and temperature. Thermodynamics also considers other quantities that are not physical properties, such as mass flow rates and energy transfers by work and heat. Energy forms Fluids and solids can possess several forms of energy. All fluids possess energy due to their temperature and this is referred to as ‘internal energy’. They will also possess ‘ potential energy’ (PE) due to distance (z) above a datum level and if the fluid is moving at a velocity (v), it will also possess ‘kinetic energy’. If the fluid is pressurised, it will possess ‘flow energy’ (FE). Pressure and temperature are the two governing factors and internal energy can be added to FE to produce a single property called ‘enthalpy’. Internal energy The molecules of a fluid possess both kinetic energy (KE) and PE relative to an internal datum. Generally, this is regarded simply as the energy due to its temperature and the change in internal energy in a fluid that undergoes a temperature change is given by ΔU = mcΔT The total internal energy is denoted by the symbol ‘U’, which has values of J, kJ or MJ; also the specific internal energy ‘u’ has the values of kJ/kg. -

The Aircraft Propulsion the Aircraft Propulsion
THE AIRCRAFT PROPULSION Aircraft propulsion Contact: Ing. Miroslav Šplíchal, Ph.D. [email protected] Office: A1/0427 Aircraft propulsion Organization of the course Topics of the lectures: 1. History of AE, basic of thermodynamic of heat engines, 2-stroke and 4-stroke cycle 2. Basic parameters of piston engines, types of piston engines 3. Design of piston engines, crank mechanism, 4. Design of piston engines - auxiliary systems of piston engines, 5. Performance characteristics increase performance, propeller. 6. Turbine engines, introduction, input system, centrifugal compressor. 7. Turbine engines - axial compressor, combustion chamber. 8. Turbine engines – turbine, nozzles. 9. Turbine engines - increasing performance, construction of gas turbine engines, 10. Turbine engines - auxiliary systems, fuel-control system. 11. Turboprop engines, gearboxes, performance. 12. Maintenance of turbine engines 13. Ramjet engines and Rocket engines Aircraft propulsion Organization of the course Topics of the seminars: 1. Basic parameters of piston engine + presentation (1-7)- 3.10.2017 2. Parameters of centrifugal flow compressor + presentation(8-14) - 17.10.2017 3. Loading of turbine blade + presentation (15-21)- 31.10.2017 4. Jet engine cycle + presentation (22-28) - 14.11.2017 5. Presentation alternative date Seminar work: Aircraft engines presentation A short PowerPoint presentation, aprox. 10 minutes long. Content of presentation: - a brief history of the engine - the main innovation introduced by engine - engine drawing / cross-section - -
![Chemical Kinetics [A][B] [D] [C] [A][B] [B] [A] K R Dt D Dt D K R Dt D Dt D](https://docslib.b-cdn.net/cover/8501/chemical-kinetics-a-b-d-c-a-b-b-a-k-r-dt-d-dt-d-k-r-dt-d-dt-d-948501.webp)
Chemical Kinetics [A][B] [D] [C] [A][B] [B] [A] K R Dt D Dt D K R Dt D Dt D
Chemical kinetics (Nazaroff & Alvarez-Cohen, Section 3.A.2) This is the answer to what happens when chemical equilibrium is not reached. For example, take the two-way reaction A + B ↔ C + D As A and B come into contact with each other, they start to react with each other, and the reaction rate can be expressed as Rforward = kf [A] [B]. This expression reflects the fact that the more of A and B there is, the more encounters occur, and the more reactions take place. This rate depletes the amounts of both A and B, and generates amounts of C and D: d[A] d[B] R k [A][B] dt dt forward f d[C] d[D] R k [A][B] dt dt forward f But, at the same time, C and D come in contact, too, and carry their own, reverse reaction, at a rate proportional to their amounts Rreverse = kr [C] [D] . This rate depletes the amounts of both C and D, and adds to the amounts of A and B: d[A] d[B] R k [C][D] dt dt reverse r d[C] d[D] R k [C][D] dt dt reverse r Because the two reactions occur simultaneously, we subtract the rate of one from the other: d[A] d[B] R R k [A][B] k [C][D] dt dt forward reverse f r d[C] d[D] R R k [A][B] k [C][D] dt dt forward reverse f r 1 Finally, in a system with open boundaries, we can add the imports and exports: d[A] V Qin [A]in Qout [A]out krV[C][D] kfV[A][B] dt inlets outlets d[B] V Qin [B]in Qout [B]out krV[C][D] kfV[A][B] dt inlets outlets d[C] V Qin [C]in Qout [C]out kfV[A][B] krV[C][D] dt inlets outlets d[D] V Qin [D]in Qout [D]out kfV[A][B] krV[C][D] dt inlets outlets imports exports sources sinks where V is the volume of the system for which the budget is written. -

Carnot Cycles
CARNOT CYCLES Sadi Carnot was a French physicist who proposed an “ideal” cycle for a heat engine in 1824. Historical note – the idea of an ideal cycle came about because engineers were trying to develop a steam engine (a type of heat engine) where they could reject (waste) a minimal amount of heat. This would produce the best efficiency since η = 1 – (QL/QH). Carnot proposed that a cycle comprised of completely (internally and externally) reversible processes would give the maximum amount of net work for a given heat input, since the work done by a system in a reversible (ideal) process is always greater than that in an irreversible (real) process. THE CARNOT HEAT ENGINE CYCLE CONSISTS OF FOUR REVERSIBLE PROCESSES IN A SEQUENCE: 1 Æ 2: Reversible isothermal expansion. Heat transfer from HTR (+) and boundary work (+) occur in closed system 2 Æ 3: Reversible adiabatic expansion Work output (+), but no heat transfer 3 Æ 4: Reversible isothermal compression Heat transfer (-) and boundary work (-) occur in closed system 4 Æ 1: Reversible adiabatic compression Work input (-), but no heat transfer AND Wout >>> Win 1 P-V DIAGRAM FOR CARNOT HEAT ENGINE CYCLE P 1 2 4 3 Showing net work is POSITIVE. V A useful example of an isothermal expansion is boiling (vaporization) at a constant pressure in a device such as a piston-cylinder. Similarly, an example of an isothermal compression is condensation at a constant pressure in a piston-cylinder. Also, heat transfer can only occur in processes 1 Æ 2 and 3 Æ4. 1 Æ 2: since work is positive (expansion) and Δu is positive (e.g., boiling) then heat transfer is positive (input from HTR).