Chapter 7 BOUNDARY WORK
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Recitation: 3 9/18/03
Recitation: 3 9/18/03 Second Law ² There exists a function (S) of the extensive parameters of any composite system, defined for all equilibrium states and having the following property: The values assumed by the extensive parameters in the absence of an internal constraint are those that maximize the entropy over the manifold of constrained equilibrium states. ¡¢ ¡ ² Another way of looking at it: ¡¢ ¡ U1 U2 U=U1+U2 S1 S2 S>S1+S2 ¡¢ ¡ Equilibrium State Figure 1: Second Law ² Entropy is additive. And... � ¶ @S > 0 @U V;N::: ² S is an extensive property: It is a homogeneous, first order function of extensive parameters: S (¸U; ¸V; ¸N) =¸S (U; V; N) ² S is not conserved. ±Q dSSys ¸ TSys For an Isolated System, (i.e. Universe) 4S > 0 Locally, the entropy of the system can decrease. However, this must be compensated by a total increase in the entropy of the universe. (See 2) Q Q ΔS1=¡ ΔS2= + T1³ ´ T2 1 1 ΔST otal =Q ¡ > 0 T2 T1 QuasiStatic Processes A Quasistatic thermodynamic process is defined as the trajectory, in thermodynamic space, along an infinite number of contiguous equilibrium states that connect to equilibrium states, A and B. Universe System T2 T1 Q T1>T2 Figure 2: Local vs. Total Change in Energy Irreversible Process Consider a closed system that can go from state A to state B. The system is induced to go from A to B through the removal of some internal constraint (e.g. removal of adiabatic wall). The systems moves to state B only if B has a maximum entropy with respect to all the other accessible states. -
Polytropic Processes
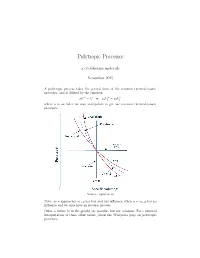
Polytropic Processes a.cyclohexane.molecule November 2015 A polytropic process takes the general form of the common thermodynamic processes, and is defined by the equation n n n pV = C or p1V1 = p2V2 where n is an index we may manipulate to get our common thermodynamic processes. Source: nptel.ac.in Note: as n approaches 1, p has less and less influence; when n = 1, p has no influence and we thus have an isobaric process. Other n values (x in the graph) are possible, but not common. For a physical interpretation of these other values, check the Wikipedia page on polytropic processes. Because polytropic processes are general representations of thermodynamic pro- cesses, any quantities we calculate for a polytropic process will be a general equation for that quantity in all other thermodynamic processes. We proceed to find the work and heat associated with a polytropic process. We start with the definition of work: Z V2 w = − p dV V1 The polytropic equation pV n = C allows us to manipulate our formula for work in the following manner, as long as n 6= 1 V V Z 2 C Z 2 C V C V w = − dV = −C V −n dV = − V 1−n 2 = V 1−n 2 n V1 V1 V1 V V1 1 − n n − 1 Evaluating this expression, C CV 1−n − CV 1−n w = V 1−n − V 1−n = 2 1 n − 1 2 1 n − 1 n n We let the first C be p2V2 and the second C be p1V1 , such that the exponents cancel to obtain p V nV 1−n − p V nV 1−n p V − p V R(T − T ) w = 2 2 2 1 1 1 = 2 2 1 1 = 2 1 n − 1 n − 1 n − 1 where the last equality follows from the ideal gas law pV = RT and our deriva- tion for the work of a polytropic process is complete. -

MECH 230 – Thermodynamics 1 Final Workbook Solutions
MECH 230 – Thermodynamics 1 Final Workbook Solutions CREATED BY JUSTIN BONAL MECH 230 Final Exam Workbook Contents 1.0 General Knowledge ........................................................................................................................... 1 1.1 Unit Analysis .................................................................................................................................. 1 1.2 Pressure ......................................................................................................................................... 1 2.0 Energies ............................................................................................................................................ 2 2.1 Work .............................................................................................................................................. 2 2.2 Internal Energy .............................................................................................................................. 3 2.3 Heat ............................................................................................................................................... 3 2.4 First Law of Thermodynamics ....................................................................................................... 3 3.0 Ideal gas ............................................................................................................................................ 4 3.1 Universal Gas Constant ................................................................................................................. -

3. Thermodynamics Thermodynamics
Design for Manufacture 3. Thermodynamics Thermodynamics • Thermodynamics – study of heat related matter in motion. • Ma jor deve lopments d uri ng 1650 -1850 • Engineering thermodynamics mainly concerned with work produc ing or utili si ng machi nes such as - Engines, - TbiTurbines and - Compressors together w ith th e worki ng sub stances used i n th e machi nes. • Working substance – fluids: capable of deformation, energy transfer • Air an d s team are common wor king sub st ance Design and Manufacture Pressure F P = A Force per unit area. Unit: Pascal (Pa) N / m 2 1 Bar =105 Pa 1 standard atmosphere = 1.01325 Bar 1 Bar =14.504 Psi (pound force / square inch, 1N= 0.2248 pound force) 1 Psi= 6894.76 Pa Design and Manufacture Phase Nature of substance. Matter can exists in three phases: solid, liquid and gas Cycle If a substance undergoes a series of processes and return to its original state, then it is said to have been taken through a cycle. Design for Manufacture Process A substance is undergone a process if the state is changed by operation carried out on it Isothermal process - Constant temperature process Isobaric process - Constant pressure process Isometric process or isochoric process - Constant volume process Adiabatic Process No heat is transferred, if a process happens so quickly that there is no time to transfer heat, or the system is very well insulated from its surroundings. Polytropic process Occurs with an interchange of both heat and work between the system and its surroundings E.g. Nonadiabatic expansion or compression -

Heat Transfer During the Piston-Cylinder Expansion of a Gas
AN ABSTRACT OF THE THESIS OF Matthew Jose Rodrigues for the degree of Master of Science in Mechanical Engineering presented on June 5, 2014. Title: Heat Transfer During the Piston-Cylinder Expansion of a Gas Abstract approved: ______________________________________________________ James A. Liburdy The expansion of a gas within a piston-cylinder arrangement is studied in order to obtain a better understanding of the heat transfer which occurs during this process. While the situation of heat transfer during the expansion of a gas has received considerable attention, the process is still not very well understood. In particular, the time dependence of heat transfer has not been well studied, with many researchers instead focused on average heat transfer rates. Additionally, many of the proposed models are not in agreement with each other and are usually dependent on experimentally determined coefficients which have been found to vary widely between test geometries and operating conditions. Therefore, the expansion process is analyzed in order to determine a model for the time dependence of heat transfer during the expansion. A model is developed for the transient heat transfer by assuming that the expansion behaves in a polytropic manner. This leads to a heat transfer model written in terms of an unknown polytropic exponent, n. By examining the physical significance of this parameter, it is proposed that the polytropic exponent can be related to a ratio of the time scales associated with the expansion process, such as a characteristic Peclet number. Experiments are conducted to test the proposed heat transfer model and to establish a relationship for the polytropic exponent. -

Thermodynamic Analysis of an Irreversible Maisotsenko Reciprocating Brayton Cycle
entropy Article Thermodynamic Analysis of an Irreversible Maisotsenko Reciprocating Brayton Cycle Fuli Zhu 1,2,3, Lingen Chen 1,2,3,* and Wenhua Wang 1,2,3 1 Institute of Thermal Science and Power Engineering, Naval University of Engineering, Wuhan 430033, China; [email protected] (F.Z.); [email protected] (W.W.) 2 Military Key Laboratory for Naval Ship Power Engineering, Naval University of Engineering, Wuhan 430033, China 3 College of Power Engineering, Naval University of Engineering, Wuhan 430033, China * Correspondence: [email protected] or [email protected]; Tel.: +86-27-8361-5046; Fax: +86-27-8363-8709 Received: 20 January 2018; Accepted: 2 March 2018; Published: 5 March 2018 Abstract: An irreversible Maisotsenko reciprocating Brayton cycle (MRBC) model is established using the finite time thermodynamic (FTT) theory and taking the heat transfer loss (HTL), piston friction loss (PFL), and internal irreversible losses (IILs) into consideration in this paper. A calculation flowchart of the power output (P) and efficiency (η) of the cycle is provided, and the effects of the mass flow rate (MFR) of the injection of water to the cycle and some other design parameters on the performance of cycle are analyzed by detailed numerical examples. Furthermore, the superiority of irreversible MRBC is verified as the cycle and is compared with the traditional irreversible reciprocating Brayton cycle (RBC). The results can provide certain theoretical guiding significance for the optimal design of practical Maisotsenko reciprocating gas turbine plants. Keywords: finite-time thermodynamics; irreversible Maisotsenko reciprocating Brayton cycle; power output; efficiency 1. Introduction The revolutionary Maisotsenko cycle (M-cycle), utilizing the psychrometric renewable energy from the latent heat of water evaporating, was firstly provided by Maisotsenko in 1976. -
Quantum Mechanics Polytropic Process
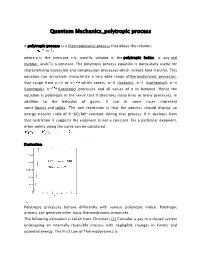
Quantum Mechanics_polytropic process A polytropic process is a thermodynamic process that obeys the relation: where p is the pressure, v is specific volume, n, the polytropic index, is any real number, andC is a constant. The polytropic process equation is particularly useful for characterizing expansion and compression processes which include heat transfer. This equation can accurately characterize a very wide range ofthermodynamic processes, that range from n<0 to n= which covers, n=0 (Isobaric), n=1 (Isothermal), n=γ (Isentropic), n= (Isochoric) processes and all values of n in between. Hence the equation is polytropic in the sense that it describes many lines or many processes. In addition to the behavior of gases, it can in some cases represent some liquids and solids. The one restriction is that the process should display an energy transfer ratio of K=δQ/δW=constant during that process. If it deviates from that restriction it suggests the exponent is not a constant. For a particular exponent, other points along the curve can be calculated: Derivation Polytropic processes behave differently with various polytropic indice. Polytropic process can generate other basic thermodynamic processes. The following derivation is taken from Christians.[1] Consider a gas in a closed system undergoing an internally reversible process with negligible changes in kinetic and potential energy. The First Law of Thermodynamics is Define the energy transfer ratio, . For an internally reversible process the only type of work interaction is moving boundary work, given by Pdv. Also assume the gas is calorically perfect (constant specific heat) so du = cvdT. The First Law can then be written (Eq. -

Physico-Mathematical Model of a Hot Air Engine Using Heat from Low-Temperature Renewable Sources of Energy
U.P.B. Sci. Bull., Series D, Vol. 78, Iss. D, 2016 ISSN 1223-7027 PHYSICO-MATHEMATICAL MODEL OF A HOT AIR ENGINE USING HEAT FROM LOW-TEMPERATURE RENEWABLE SOURCES OF ENERGY Vlad Mario HOMUTESCU1, Marius-Vasile ATANASIU2, Carmen-Ema PANAITE3, Dan-Teodor BĂLĂNESCU4 The paper provides a novel physico-mathematical model able to estimate the maximum performances of a hot air Ericsson-type engine - which is capable to run on renewable energy, such as solar energy, geothermal energy or biomass. The computing program based on this new model allows to study the influences of the main parameters over the engine performances and to optimize their values. Keywords: Ericsson-type engine, renewable energy, theoretical model, maximum performances. 1. Introduction In the last decades we witnessed an extraordinary development of numerous engines and devices capable of transforming heat into work [1]. The field of the “unconventional” engines has risen again into the attention of the researchers and of the investors. Several former abandoned or marginal technical solutions of hot air engines were reanalyzed by researchers - as the Ericsson-type reciprocating engines [2], Stirling engines or Stirling-related engines [1], [3] and the Vuilleumier machine. The Ericsson-type hot air engine is an external combustion heat engine with pistons (usually, with pistons with reciprocating movement) and with valves. An Ericsson-type engine functional unit comprises two pistons. The compression process takes place inside a compression cylinder, and the expansion process takes place inside an expansion cylinder. Heat is added to the cycle inside a heater. Sometimes a heat recuperator or regenerator is used to recover energy from the exhaust gases, fig. -

Chapter 11 ENTROPY
Chapter 11 ENTROPY There is nothing like looking, if you want to find something. You certainly usually find something, if you look, but it is not always quite the something you were after. − J.R.R. Tolkien (The Hobbit) In the preceding chapters, we have learnt many aspects of the first law applications to various thermodynamic systems. We have also learnt to use the thermodynamic properties, such as pressure, temperature, internal energy and enthalpy, when analysing thermodynamic systems. In this chap- ter, we will learn yet another thermodynamic property known as entropy, and its use in the thermodynamic analyses of systems. 250 Chapter 11 11.1 Reversible Process The property entropy is defined for an ideal process known as the re- versible process. Let us therefore first see what a reversible process is all about. If we can execute a process which can be reversed without leaving any trace on the surroundings, then such a process is known as the reversible process. That is, if a reversible process is reversed then both the system and the surroundings are returned to their respective original states at the end of the reverse process. Processes that are not reversible are called irreversible processes. Student: Teacher, what exactly is the difference between a reversible process and a cyclic process? Teacher: In a cyclic process, the system returns to its original state. That’s all. We don’t bother about what happens to its surroundings when the system is returned to its original state. In a reversible process, on the other hand, the system need not return to its original state. -

BASIC THERMODYNAMICS REFERENCES: ENGINEERING THERMODYNAMICS by P.K.NAG 3RD EDITION LAWS of THERMODYNAMICS
Module I BASIC THERMODYNAMICS REFERENCES: ENGINEERING THERMODYNAMICS by P.K.NAG 3RD EDITION LAWS OF THERMODYNAMICS • 0 th law – when a body A is in thermal equilibrium with a body B, and also separately with a body C, then B and C will be in thermal equilibrium with each other. • Significance- measurement of property called temperature. A B C Evacuated tube 100o C Steam point Thermometric property 50o C (physical characteristics of reference body that changes with temperature) – rise of mercury in the evacuated tube 0o C bulb Steam at P =1ice atm T= 30oC REASONS FOR NOT TAKING ICE POINT AND STEAM POINT AS REFERENCE TEMPERATURES • Ice melts fast so there is a difficulty in maintaining equilibrium between pure ice and air saturated water. Pure ice Air saturated water • Extreme sensitiveness of steam point with pressure TRIPLE POINT OF WATER AS NEW REFERENCE TEMPERATURE • State at which ice liquid water and water vapor co-exist in equilibrium and is an easily reproducible state. This point is arbitrarily assigned a value 273.16 K • i.e. T in K = 273.16 X / Xtriple point • X- is any thermomertic property like P,V,R,rise of mercury, thermo emf etc. OTHER TYPES OF THERMOMETERS AND THERMOMETRIC PROPERTIES • Constant volume gas thermometers- pressure of the gas • Constant pressure gas thermometers- volume of the gas • Electrical resistance thermometer- resistance of the wire • Thermocouple- thermo emf CELCIUS AND KELVIN(ABSOLUTE) SCALE H Thermometer 2 Ar T in oC N Pg 2 O2 gas -273 oC (0 K) Absolute pressure P This absolute 0K cannot be obtatined (Pg+Patm) since it violates third law. -

Problem 3.130
PROBLEM 3.130 PROBLEM 3.117 3 o 2 As shown in Fig. P3.117, 20 ft of air at T1 = 600 R, 100 lbf/in. undergoes a polytropic expansion to a final pressure of 51.4 lbf/in.2 The process follows pV1.2 = constant. The work is W = 194.34 Btu. Assuming ideal gas behavior for the air, and neglecting kinetic and potential energy effects, determine (a) the mass of air, in lb, and the final temperature, in oR. (b) the heat transfer, in Btu. KNOWN: Air undergoes a polytropic process in a piston-cylinder assembly. The work is known. FIND: Determine the mass of air, the final temperature, and the heat transfer. SCHEMATIC AND GIVEN DATA: o T1 = 600 R Q 2 p1 = 100 lbf/in. 3 V1 = 20 ft Air 2 P2 = 51.4 lbf/in. W = 194.34 Btu 1.2 pV = constant pv1.2 = constant p 1 100 . T1 ENGINEERING MODEL: 1. The air is a closed system. 2. Volume change is the only work mode. 3. The process is 1.2 2 polytropic, with pV = constant and W = 194.34 Btu. 51.4 . T2 4. Kinetic and potential energy effects can be neglected. ANALYSIS: (a) The mass is determined using the ideal gas equation of state. v m = = = 9.00 lb 1.2 To get the final temperature, we use the polytropic process, pV = constant, to evaluate V2 as follows. 3 3 V2 = = (20 ft ) = 34.83 ft Now PROBLEM 3.117 (CONTINUED) o T2 = = = 537 R Alternative solution for T2 1.2 The work for the polytropic process can be evaluated using W = . -

Isothermal Heating: Purist and Utilitarian Views
European Journal of Physics Eur. J. Phys. 39 (2018) 045103 (11pp) https://doi.org/10.1088/1361-6404/aac234 Isothermal heating: purist and utilitarian views Harvey S Leff1,2,4 and Carl E Mungan3 1 California State Polytechnic University-Pomona, United States of America 2 Reed College, United States of America 3 Physics Department, US Naval Academy, Annapolis, MD 21402, United States of America E-mail: [email protected] and [email protected] Received 8 December 2017, revised 7 April 2018 Accepted for publication 3 May 2018 Published 23 May 2018 Abstract The common thermodynamics example of reversible isothermal heating at temperature T requires the gas to maintain contact with a constant temperature reservoir at the same temperature as the gas. This is inconsistent with the often used view that heat is an energy transfer from higher to lower temperature. We discuss two ways to deal with this dichotomy, using what we call purist and utilitarian views, emphasizing the roles of language and logic in each. We suggest a way for teachers to address this issue in the classroom, and end with a review of related challenges to physics teachers associated with the terms heat and heating. Keywords: heat, thermodynamics, language, isothermal, reversible 1. Introduction Thermodynamics is fraught with nuances and subtleties that can be challenging for students. This makes the subject difficult to teach. Consider a gas that is in thermal contact with a reservoir at temperature T and is in thermodynamic equilibrium. Let us examine a reversible isothermal volume change where the term isothermal implies a constant, uniform gas temperature throughout the process.